Introduction
The main emissions of pollutants into the atmosphere in the field of power engineering are from thermal power plants. The most significant types of air pollutant emissions from combustion of fossil fuels at power plants are sulfur dioxide, nitrogen oxides, carbon monoxide and solid fuel ash, which are accepted as marker substances, as well as the greenhouse gas - carbon dioxide CO2 [1; 2, p. 109-119; 3]. Other pollutants – benz(a)pyrene, soot, solid (coke) particles of unburned fuel, which are products of fuel underburning, are formed in insignificant amounts, as a rule, during short-term operation of power plants in transient modes and do not have a noticeable impact on public health and the environment [4, 5].
The main mass of pollutant emissions into the atmosphere falls on large fuel oil and especially coal-fired thermal power plants [6; 7; 8; 9, p. 1-19].
Flue gas composition
Emissions from power plants operating on traditional fuels (gasoline, kerosene, diesel fuel, fuel oil, coal, natural gas, etc.) have a serious impact on the environment and human health. Among the main problems caused by emissions from such plants are the following [10; 11; 12, p. 32-35; 13; 14; 15]:
- Air pollution: emissions from thermal power plants include various harmful substances such as sulfur dioxide, nitrogen dioxide, heavy metals and others. These substances can cause asthma, chronic respiratory diseases, cancer and other serious illnesses.
- Climate Change: Emissions of greenhouse gases such as carbon dioxide, methane, and others are increasing the greenhouse effect and changing the climate conditions on the planet. This can lead to global warming, changing sea levels and other negative effects.
- Water and soil pollution: power plant emissions can contain toxic substances that enter the soil and water sources, polluting them and having harmful effects on ecosystems and animal and human health.
- Threat to biodiversity: power plant emissions can cause the decline and extinction of a number of animal and plant species, leading to an imbalance in ecosystems and threatening biodiversity. Introduction
The main emissions of pollutants into the atmosphere in the field of power engineering are from thermal power plants. The most significant types of air pollutant emissions from combustion of fossil fuels at power plants are sulfur dioxide, nitrogen oxides, carbon monoxide and solid fuel ash, which are accepted as marker substances, as well as well as the greenhouse gas – carbon dioxide CO2 [1; 2, p. 109-119; 3]. Other pollutants – benz(a)pyrene, soot, solid (coke) particles of unburned fuel, which are products of fuel underburning, are formed in insignificant amounts, as a rule, during short-term operation of power plants in transient modes and do not have a noticeable impact on public health and the environment [4, 5].
The main mass of pollutant emissions into the atmosphere falls on large fuel oil and especially coal-fired thermal power plants [6; 7; 8; 9, p. 1-19]. The values of specific pollutant emissions are influenced by various factors [16, p. 469-474; 17]. These include steam capacity of boiler plants, their technical condition and service life, the structure and quality of combusted fuel, the presence of measures to suppress the formation of nitrogen oxides, the technical condition of ash collectors, the degree of gas purification in them, etc.
The composition of emissions depends on the type of combusted organic fuel. The working mass of solid organic fuel consists of carbon, hydrogen, oxygen, nitrogen, sulfur, moisture and ash. At complete combustion of fuel, carbon dioxide, water vapor, sulfur oxides (SO2, SO3) and ash are formed. Of the listed components of combustion products, sulfur oxides and ash are among the toxic ones [18, 19]. At high temperatures, partial oxidation of air and fuel nitrogen with formation of nitrogen oxides (NO, NO2) takes place in the flame core of the flame of furnace chambers of large capacity boilers. If the combustion zone receives less air than required by the stoichiometry of the combustion reaction, or when combustion is carried out at low temperature, in addition to the products of complete combustion, incomplete combustion products are formed – CO, CH4, C2H4, etc., as well as carcinogenic substances. Products of incomplete combustion are very harmful. The highest ash content is found in oil shale and lignite, as well as in some types of hard coal. Their composition depends on the deposit. Liquid fuels have a small ash content, but the same emissions of carbon monoxides and sulfur oxides, because fuel oils used as fuel contain 2 and more % of sulfur [20, 21, 22]. In general, flue gases from fuel oil combustion contain nitrogen oxides, vanadium and sodium compounds, gaseous products of incomplete combustion. Natural gas is an ashless fuel. But when burning natural gas, flue emissions also contain sulfur oxides and nitrogen oxides [23, p. 287-291; 24]. And the largest amount of nitrogen oxides is formed during combustion of liquid fuels.
The global trend of low-carbon energy transition is directed toward the transition to more environmentally friendly energy sources. However, for various reasons, there are enough thermal power plants burning solid fuels in the world. In 2023, more than 69.5 GW of coal-fired power plants were put into operation all over the planet, which is three times more than decommissioned (21.12 GW) (fig. 1) [25, 26].
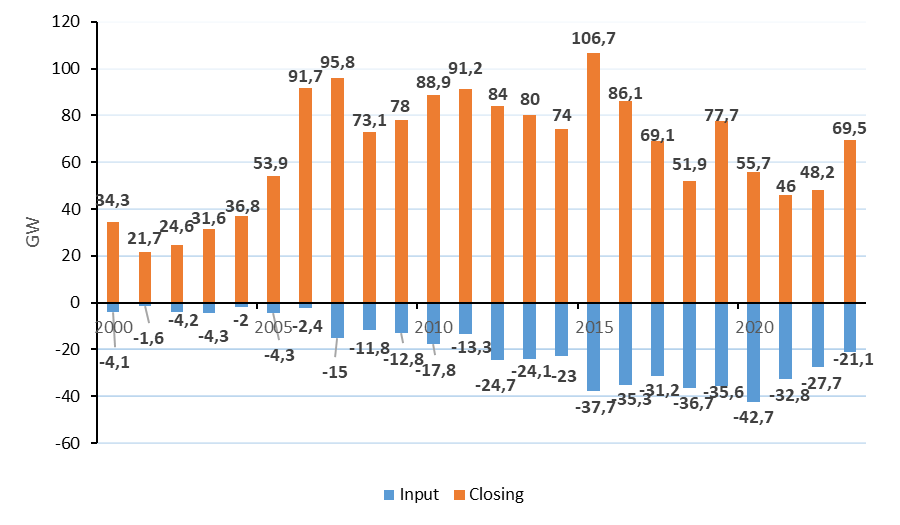
Fig. 1. Volumes of input and output of coal-fired generation capacity in the world, GW
For example, a 24,000 MW power plant consumes about 20,000 tons of coal per day, while emitting into the atmosphere 680 tons of CO2 and CO3, 200 tons of nitrogen oxides, 120–240 tons of particulate matter, which includes ash, soot, dust. And all this every day.
Impact of smoke emissions on the environment and people
Emissions from power plants have a harmful impact on the entire biosphere. And emissions from the smokestacks of thermal power plants are estimated at a distance of 20 to 50 kilometers. The constituents of these emissions have various effects on soil, plants, water bodies, and people [27].
SO2:
SO2 has toxic effects on plants [28, p. 523-529; 29] by destroying chlorophyll and consequently damaging leaf and needle surfaces. Conifers that do not shed leaves are more susceptible to SO2. Thus, SO2 concentration in the air of 0.23-0.32 mg/m3 leads to plant death in 2–3 years.
Formation of toxic fogs in the atmosphere polluted with SO2 is dangerous by the probability of occurrence of chronic non-specific diseases in people, such as atherosclerosis, coronary and degenerative heart diseases, chronic bronchitis, emphysema, bronchial asthma, etc. At SO2 concentrations of 0.08-0.10 mg-m3, symptoms of respiratory deterioration may appear, and at concentrations of 0.25-0.5 mg-m3, the condition of patients with pulmonary diseases worsens until death [30, 31].
When exposed to sunlight and tiny dust particles, SO2 oxidizes to SO3 and forms sulfuric acid when it interacts with air moisture.
NOx:
Nitrogen oxides are also the most toxic to humans. The toxicity of coal and fuel oil combustion products is determined by nitrogen oxides by 40-50%, and natural gas by 90-95%. They have a pronounced irritating effect, especially on the mucous membrane of the eye, are able to penetrate deep into the lungs, causing damage to the alveolar epithelium and bronchi [32; 33, p. 133-141].
At NO2 concentrations of 34-6 mg/m3, acute plant damage occurs, and even concentrations less than 2 mg/m3 result in reduced plant growth.
The dissociation reaction of NO2 provokes many secondary reactions, the appearance of free radicals, ozone formation, and polymerization. Photochemical reactions with NO2 occur in stages, resulting in the continued formation of nitrogen oxides as the smoke plume travels. Moreover, NO2 is 3-3.5 times more toxic than NO [34, p. 74-83; 35, p. 541-558].
Water vapors:
The sources of water vapor in thermal power plants are cooling towers and chimneys. In flue gases, the most water vapor is produced by combustion of natural gas.
Water vapors themselves are not harmful to humans, but in large quantities they lead to fog formation, ice formation on structures and roads near power plants, ice build-up on wires and to the breakage of power lines. Water vapor also contributes to the formation of sulfuric acid vapor and photochemical fog – smog.
Emissions from smokestacks and wastewater discharges from fossil fuel-fired power plants affect water bodies. Depending on the concentration, toxic compounds can lead to pH imbalance, negative impact on biocenosis and even death of hydrobionts.
Thus, it is necessary to take measures to minimize emissions from fossil fuel combustion by creating environmentally friendly power plants and modernizing the already operating plants.
Methods of reducing emissions into the atmosphere
Reduction of ash emissions
The content of ash as well as sulphur dioxide is largely determined by the composition of the fuel itself. Concentrations of these compounds in solid fuels range from 12720 to 81390 mg/m3 for ash and from 620 to 7260 mg/m3 for SO2. Primarily, when solid fuels such as coal, lignite and oil shale are burned, the mineral part is mainly transferred to ash [36]. Here too, the reduction of ash particle emissions should be considered. Ash content of fuel depends on its deposit and reaches almost 60 %. The volume of ash particles emission from boiler furnaces depends on the type of furnace device, fuel ash content, fuel consumption, gas cleaning efficiency. Accordingly, at a given fuel consumption and availability of fuel with a certain ash content, it is necessary to pay attention to the furnace and gas cleaning.
The share of ash particles removal from pulverized coal furnaces can be estimated by the removal coefficient α. The values of this coefficient for different types of furnaces are given in table [37].
Table
Coefficient α for different types of pulverized coal furnaces
| Furnace type | α |
| Chamber with solid bottom ash removal | 0.95 |
| Open with liquid bottom ash removal | 0.7-0.85 |
| Semi-open with liquid bottom ash removal | 0.6-0.8 |
| Two-chamber | 0.5-0.6 |
| With vertical preheaters | 0.2-0.4 |
| With horizontal cyclone preheaters | 0.1-0.15 |
The furnace with horizontal cyclone preheaters has the lowest ash removal. In addition, this design is characterized by a reduction of nitrogen oxide formation. Even more reduces the reduction of ash particles emission by gasification of solid fuel. The next stage of ash emission reduction is ash collection of particles with the size from 1 mm to 1 micron. For this purpose cyclone ash collectors, wet inertial ash collectors, electrostatic precipitators, fabric filters are used [38, 39].
The principle of operation of dry-type ash collectors consists in the separation of combustion products from the gas-air flow under the influence of inertial forces (fig. 2) [39; 40, p. 19-26]. The mechanical basis of such plants does not allow influencing the chemical composition of the purified flow.
The principle of operation of liquid plants consists in the passage of the purified flow through a liquid barrier. Depending on the type of absorber it can be represented by a microaerosol, foam layer, liquid film or fluidized bed.
Efficiency of ash collectors is determined by their efficiency (gas cleaning coefficient). The total efficiency is the ratio of the mass flow rate of the pollutant captured by the unit to its mass flow rate at the inlet to the unit [41]:
![]() , (1)
, (1)
![]() – mass flow rate of pollutant captured by the unit,
– mass flow rate of pollutant captured by the unit,
![]() – mass flow rate at the inlet to the apparatus.
– mass flow rate at the inlet to the apparatus.
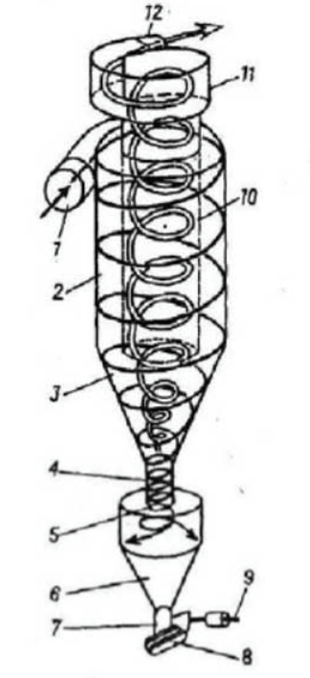
Fig. 2. Cyclone ash collector: 1 – inlet pipe, 2 – cyclone body, 3 – cone, 4 – dust outlet pipe, 5 – dust outlet opening, 6 – dust hopper, 7 – dust outlet pipe, 8 – dust valve, 9 – valve lever, 10 – dust pipe, 11 – snail, 12 – exhaust pipe
At present the required ash emission reduction efficiency for the European Union Directive is 99.8%.
Efficiency of ash collection in battery cyclone is 75–85%, in liquid plants – 95–97%. For removal of ash particles with the size less than 10 microns it is effective to use an electrostatic precipitator, the principle of operation of which is based on the ability of ash particles to receive an electric charge and be attracted to the electrode with the opposite charge. The efficiency of electrostatic precipitators can reach 99,95%. The disadvantages of ESPs are high sensitivity of the process to deviations from the specified technological mode, as well as to minor mechanical defects of the internal equipment, which may result from insufficiently careful installation works or unskilled maintenance during operation [42].
Fabric filters can be used in the form of cylindrical bags or flat frames. In terms of capital and operating costs, fabric filters and electrostatic precipitators are approximately the same, but unlike electrostatic precipitators, fabric filters are easier to operate with efficiency up to 99.9%, so they have become widespread. Possibilities of their use, especially bag filters, are expanding due to creation of new temperature-resistant and resistant to aggressive gases fabrics [43, 44].
Reduction of emissions of sulphur compounds
Reduction of sulfur compound emissions during fuel combustion at thermal power plants is possible by the following methods:
- fuel purification from sulfur compounds before fuel combustion.
- sulfur binding during combustion.
- Flue gas purification.
In some countries, such as Japan and the USA, for preliminary purification of fuel from sulfur they use mainly either catalytic hydrogenation with the release of sulfur in the form of hydrogen sulfide with its subsequent reduction to elemental sulfur, or vacuum distillation [45]. It is somewhat more difficult to remove organic sulfur, but work is being done in this direction as well. For example, patent C10L9/02 - Treating solid fuels to improve their combustion by chemical means describes a method of removing organic sulfur from high-sulfur coal using supercritical fluids.
The most widespread way of sulfur binding in the process of combustion is burning of coals in fluidized bed in special furnaces. In addition to sulfur oxides, emissions of nitrogen oxides can be significantly reduced by this method [46].
Removal of sulfur oxides and sulfur dioxide from flue gases is possible by various methods [47]:
- lime or soda cleaning.
- wet limestone method.
- magnesite method.
- ammonia-nitric acid method.
- ammonia-autoclave method.
- using WSA (Wet gas Sulphuris Asid) technology and others.
Some methods allow to obtain marketable products from the separated components. For example, ammonia-nitric acid method consists in purification of waste gases from sulfur dioxide by sulfite-bisulfite solution with subsequent decomposition of the obtained nitric acid solutions:
(NH4)2HSO3 + 2HNO3 → 2NH4NO3 + SO2 + H2O;
NH4HSO3 + HNO3 → NH4NO3 + SO2 + H2O.
As a result of these reactions, commercial sulfur dioxide and ammonium nitrate used as nitrogen fertilizer are formed. In the ammonia-autoclave method, the decomposition of sulfite-bisulfite solutions is carried out not by acids, but by heating in an autoclave to 140-150 ° C. In this acidic environment, the decomposition of ammonium salts occurs. In this case in acidic environment decomposition of ammonium salts takes place
2NH4НSO3 + (NH4)2SO3 → 2(NH4)2SO4 + S + H2O.
The resulting ammonium sulfate and sulfur are used as marketable products.
More than three dozen desulfurization methods have been developed to date, but 2 main groups of approaches to industrial desulfurization have been actually implemented with subsequent improvement. These are dry surface adsorption and wet full-volume absorption of sulfur-containing impurities in liquid-film scrubbers and nozzle absorbers (fig. 3) [39; 48, p. 219-228].
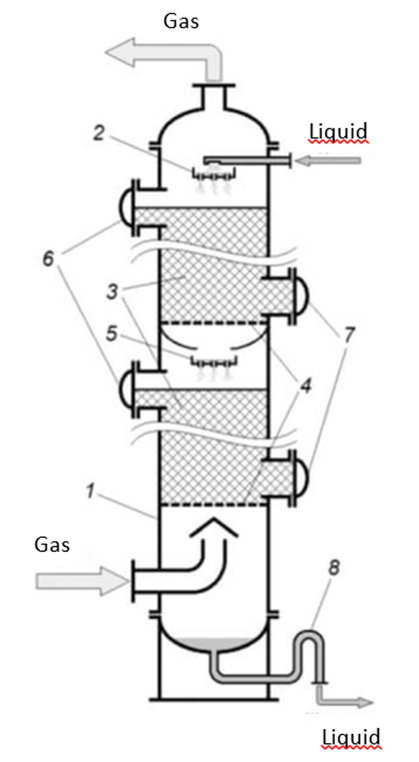
Fig. 3. Schematic diagram of the film nozzle scrubber: 1 – filter housing, 2 – distributing plate (optional), 3 – massifs of nozzle mass-exchange bodies, 4 – support grids, 5 – distributing plates (optional), 6,7 – service hatches, 8 – odor trap (optional)
Reduction of nitrogen oxide emissions
Nitrogen oxides are formed from air molecular nitrogen at a temperature of 1300 °C, from fuel nitrogen, or from the interaction of air molecular nitrogen with hydrocarbon radicals [49]. Thus, understanding the mechanisms of nitrogen oxides formation it is possible to take actions aimed at their reduction, viz:
- staged fuel combustion, which allows to reduce these emissions by 35–45%.
- staged air supply, reducing emissions by 50%.
- flue gas recirculation (33%).
- water injection into the flare core (25–44%).
By combining these methods, nitrogen oxide emissions can be reduced by up to 90%.
СO2:
The current strategy to reduce CO2 is carbon capture and storage. Capture can be organized before combustion, during combustion and after combustion. Organization of CO2 capture is more economically feasible because it does not require replacement of equipment. For this purpose such processes as adsorption, absorption, cryogenic distillation and membrane separation are used.
For example, membrane separation is widely used (figure 4). Membranes made of polymeric materials (polyacetylene, polyaniline, polyamides, polyetherimides, etc.) operate by the mechanism of solution diffusion [50, p. 34]. In facilitated membranes, CO2 transport is enhanced by interactions between carbon dioxide molecules through reversible reactions. Compared to other materials, polymeric materials can be considered as optimal materials due to many characteristics such as thermal stability, mechanical strength and chemical resistance. However, CO2 adsorption by polymer-based materials can cause swelling and plasticization problems. Non-polymeric membranes based on activated carbon, zeolites, silica and metal-organic frameworks are devoid of these drawbacks, but the cost is high. Ceramic membranes made of aluminum oxide, titanium oxide and carbon nanotubes are under research.
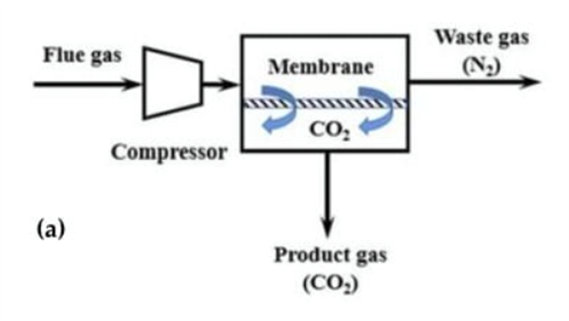
Fig. 4. One-stage process for CO2 extraction from flue gases
Reduction of water vapor emissions
Technologies are known to produce condensate from the water vapor of flue gases. Flue gas condensation is a process in which the flue gas is cooled below its dew point, and the heat released by water condensation is utilized as low-temperature heat. Flue gas condensation can cause the utilized heat to exceed the lower heating value of the original fuel and hence the efficiency exceeds 100% [51].
Flue gas cooling can be realized either directly by means of a heat exchanger or indirectly by means of a condensing scrubber. The most widespread technologies are waste heat recovery boilers.
Conclusions
In the work the influence of flue gas components, ash, sulfur oxides, nitrogen, carbon oxides, formed at combustion of organic fuel, on the environment and on a person was investigated. The review of methods allowing to reduce harmful emissions is carried out. To reduce emissions of ash particles without replacement of the main boiler equipment the optimal is ash collection from chimneys by electrostatic precipitators and fabric bag filters, allowing to catch 99,9% of ash particles. Dry surface adsorption and wet full-volume absorption of sulfur-containing impurities in liquid-film scrubbers and nozzle absorbers are really used among the methods of sulfur emission reduction. The most toxic of the considered flue gas components are nitrogen oxides. Different methods of reducing these emissions give results from 25 to 50%, while a combination can increase the efficiency up to 99.9%. The current trend in reducing carbon dioxide emissions is towards carbon dioxide capture. The actively developing direction here is the use of membrane technologies with polymeric and non-polymeric membranes. Ceramic membranes made of aluminum oxide, titanium oxide and carbon nanotubes are under research. Also given are methods of capturing water vapor that is not toxic but leads to a number of problems. Cooling of flue gases to condense water vapor can be done either directly with a heat exchanger or indirectly with a condensing scrubber. The most common technologies are utilizing waste heat recovery boilers. Modern methods also allow to utilize the heat of flue gases during flue gas cleaning and to obtain marketable products from the removed components.
.png&w=384&q=75)
.png&w=640&q=75)