Introduction
The main cause of death worldwide is cardiovascular diseases (CVDs), which killed 17.9 million people in 2019, 32% of all fatalities. It is the main cause of death in industrialised and developing countries, and lifestyle changes have increased its incidence over the past few decades [1, p. 74]. Cardiovascular diseases (CVDs) are a group of disorders of the heart and blood vessels that cause acute events like heart attacks and strokes by blocking blood flow to the heart or brain. They remain the leading cause of morbidity and mortality. Myocardial fibrosis (MF) is a common pathological outcome of cardiac injuries, characterised by the gradual buildup of extracellular matrix (ECM) proteins in fibrillar collagens that contribute to CVDs and heart failure. Fibrosis is the pathological accumulation of fibrous connective tissue, mostly collagens and fibronectin, in the heart, which is a key component of most cardiac pathological diseases [2, p. 108-119]. Sudden cardiomyocyte loss causes the most ventricular fibrotic remodelling due to the adult mammalian heart's limited regenerative potential. Acute myocardial infarction causes a rapid depletion of cardiomyocytes, leading to an inflammatory response and substitution of dead cardiac tissue with collagen scars [3]. Biomarkers help diagnose, analyse, and provide evidence of pathological conditions of cardiovascular diseases (CVDs) and their associated diseases, distinguishing between cardiac diseases, guiding therapy, and assessing cardiovascular event risk [4, p. 466-482]. Additionally, pro-fibrotic cytokines like TGF-β1 and CTGF have been linked to cardiac fibrosis. These cytokines increase ECM production and fibrosis [5]. A healthy heart only has fibroblasts. Resident fibroblasts multiply when stimulated. Fibroblasts come from endothelial, bone marrow, and epicardium cells. Cardiomyocytes enlarge and muscle fibres disorganise in response to stimuli [6, p. 1026-1036].
Many studies have linked matrix metalloproteinase 9 (MMP-9) levels to myocardial infarction (MI) mortality and left ventricular remodelling and dysfunction. MMP-9 is released by leukocytes, cardiomyocytes, fibroblasts, and endothelial cells throughout cardiac healing. MMP-9 increases inflammation and reduces it via encouraging pro-inflammatory to reparative cell transition. MMP-9 also reduces scar stiffness and neovascularization [7, p. 1566-1575]. One of the most studied MMPs, matrix metalloproteinase (MMP)-9, modulates cardiovascular disease pathological remodelling processes as inflammation and fibrosis. MMP-9 directly destroys ECM proteins and triggers cytokines and chemokines to remodel tissue [8, p. 680-686]. Many histopathological and oxidative stress indicators change with isoprenaline. The subendocardial layer, apical myocardium, left ventricle, papillary muscle, and interventricular septum show histopathological changes. Inducing myocardial infarction with isoprenaline is simple. Substance delivery can occur by subcutaneous, intraperitoneal, or intravenous methods [9, p. 433-436]. Losartan inhibits AT1 receptors. The cardiac RAS is blocked by ACE medicines, according to research. Angiotensin-converting enzyme inhibitors or blockers can improve left ventricle function, prevent shape changes, and extend lifespan in people with hypertension, heart failure, ischemic heart disease, and diabetes mellitus. In pulmonary fibrosis and other fibrotic disorders, losartan is antifibrotic [10, p. 1446-1451].
Methods and materials
Study Samples
This study employed 100 200–250g waster albino adult male rats (Rattus norvegicous). The laboratory animal home at Basra University College of Veterinary Medicine provided them. Metal-lidded plastic cages held the animals and featured drinking water and feeding spots. The cage floors were lined with wood shavings and cleaned weekly. For optimal lab settings, the lighting was 12 hours of light and 12 hours of darkness, and the room temperature was 20–25°C. Animals were provided free food and water for two weeks to acclimatise.
Twenty male rats were subcutaneously injected with 65 mg/kg.bw/day isoproterenol in 1 ml saline for five days [11, p. 1795-1802; 12, p. 1145-1152]. To detect myocardial infarction, cardiac troponin tests were performed 10 days after the initial isoproterenol dose. Tissue sections of preserved hearts were used to analyse myocardial infarction histopathology.
Study groups
The animals in this study were divided into two main groups, and each group was divided into several subgroups as follows:
Group 1; The Experimental Induction of Myocardial Infarction: this group included twenty (20) male rats that were injected subcutaneously with 65mg \kg. b.w \day isoproterenol for five days [11, p. 1795-1802; 12, p. 1145-1152].
Group 2: this group included forty-eight male rats (48), which were divided randomly into four (4) subgroups:
- Subgroup 1 negative control group included eight male rats that were injected subcutaneously with 1 ml of normal saline.
- Subgroup 2: positive control included 24 male rats were injected subcutaneously with 65 mg \kg b.w \day for five days and then housed for ten days after that, subdivision randomly into three groups:
Groupe E: 8 male rats, all animals sacrificed, and subsequently blood was collected, and hearts were preserved in formalin.
Group D30: received orally 50 mg \kg b.w \day losartan for 30 days.
Group D60: received orally 50 mg \kg bw \day losartan for 60 days.
- Subgroup 3: group B: 8 male rats received orally 50 mg \kg losartan for 55 days, then in fifty-five days injected with 65 mg \kg isoproterenol for five days (protective role of losartan).
- Subgroup 4: group A 8 male rats received orally 50 mg /kg b.w\day losartan for 60 days.
Hematological parameters, such as hemoglobin concentration, RBC count, differential WBC count, and WBC count platelets determinations
Haematological variables were assessed by an automated analyser. Scientific blood component quantification uses a two-chamber apparatus. The first chamber analyses red blood cell haemoglobin to measure white blood cells. Spectrophotometers measure haemoglobin optical density after breaking down red blood cells. Saturating a solution with Alaazoton to neutralise the charge was used to measure an electric field in the second chamber. RBCs and platelets are measured via a constant-voltage column in the room. The partial resistance blood components create to the pole voltage electric current can be used to determine their resistance.
Pathological examination
Compared to the control and treatment groups, the infraction induction group had changes in the blood vessels, the sizes, shapes, colour, and symmetry of the hearts, and pathological changes like fibrosis or spots on the heart surface.
Immunohistochemistry determinations
IHC uses a visible marker to identify tissue components utilising a specific antigen/antibody reaction. Immunohistochemistry (IHC) shows the distribution and localisation of biological components in cells and tissues. Immunostaining was done according to Michael [13, p. 109-116].
Microphotography
The histological section was imaged with an Olympus RPA light microscope using a high-resolution Sony camera, and the immunohistochemical section. Image analysis was conducted using ImageJ software VERSION 9, based on the area of protein expression.
Statistical analysis
In order to determine whether the groups under examination differed significantly from one another, the statistical analysis program SPSS (Statistical Package for the Social Sciences) was utilised.
Results
Hematological parameters levels of study groups
hematological tests revealed significant differences in white blood cell count, monocytes, and hemoglobin in group e (18.33 ± 2.56, 3.99 ± 0.48, 9.54 ± 1.09, p < 0.001) compared to control, but no significant difference in groups D30 (8.74 ± 1.15, 2.9 ± 1.49, 12.08 ± 1.14), D60 (8.08 ± 0.76, 2.56 ± 0.65, and 11.18 ± 0.7) compared to control figures (1a–1c) (table 1). Lym % levels in group e (59.91 ± 1.53) and group a (49.89 ± 10.17) showed significant differences in compression with control, while groups D30 (58.59 ± 9.47), D60 (56.54 ± 4.18), and group b (56.43 ± 5.57) showed non-significant differences (p-value > 0.001) figure (2), (table 1). R.B.C count levels differed significantly (p-value > 0.001) in groups e (6.58 ± 0.79), d30 (9.99 ± 1.5), d60 (8.79 ± 0.84), b (8.18 ± 0.98), and a (losartan: 8.1 ± 0.94) compared to the control group figure (3), (table 1). platelet levels significantly differed (p-value > 0.001) in experimental groups e (391.39 ± 61.74), D30 (279.04 ± 53.93), d60 (269.23 ± 24.98), b (309.58 ± 55.89), and a (270.05 ± 42.82) compared to control group figure (4), (table 1). Compared to the control group, granulocyte % levels in experimental groups e (42.93 ± 7.19), D30 (34.04 ± 7.56), D60 (34.7 ± 11.32), b (35.18 ± 9.82), and a (37.04 ± 10.08) exhibited no significant changes (p-value > 0.001) figure 5, (table 1).
Table 1
Effect of isoproterenol injection and losartan treatment on hematological parameters levels in different experimental groups
parameters | Control | E | D30 | D60 | B | A | LSD0.05 |
WBC | 8.69 ± 0.58 | 18.33 ± 2.56 | 8.74 ± 1.15 | 8.08 ± 0.76 | 8.88 ± 0.67 | 8.03 ± 0.7 | 80.44 <0.001 S |
Lym % | 56.54 ± 4.55 | 59.91 ± 1.53 | 58.59 ± 9.47 | 56.54 ± 4.18 | 56.43 ± 5.57 | 49.89 ± 10.17 | 2.15 < 0.077 NS |
Mon% | 1.99 ± 0.63 | 3.99 ± 0.48 | 2.9 ± 1.49 | 2.56 ± 0.65 | 2.68 ± 0.9 | 2.2 ± 0.54 | 5.45 < 0.001 S |
Gran% | 35.1 ± 8.83 | 42.93 ± 7.19 | 34.04 ± 7.56 | 34.7 ± 11.32 | 35.18 ± 9.82 | 37.04 ± 10.08 | 1.021 < 0.417 S |
R.B.C count | 8.2 ± 0.85 | 6.58 ± 0.79 | 9.99 ± 1.5 | 8.79 ± 0.84 | 8.18 ± 0.98 | 8.1 ± 0.94 | 9.586 <0.001 S |
HB | 11.18 ± 0.7 | 9.54 ± 1.09 | 12.08 ± 1.14 | 11.78 ± 0.97 | 11.25 ± 1.36 | 10.91 ± 0.87 | 5.76 <0.001 S |
PLT | 252.01 ± 31.54 | 391.39 ± 61.74 | 279.04 ± 53.93 | 269.23 ± 24.98 | 309.58 ± 55.89 | 270.05 ± 42.82 | 9.307 <0.001 S |
E: heart infarction induced by isoproterenol injection, D30: treatment for 30 days, D60: treatment for 60 days, B: protective role (lsoproterenol+losartan), A: losartan.
S: Significant difference between groups (p-value <= 0.001).
NS: Non-significant difference between groups (p-value >0.001).
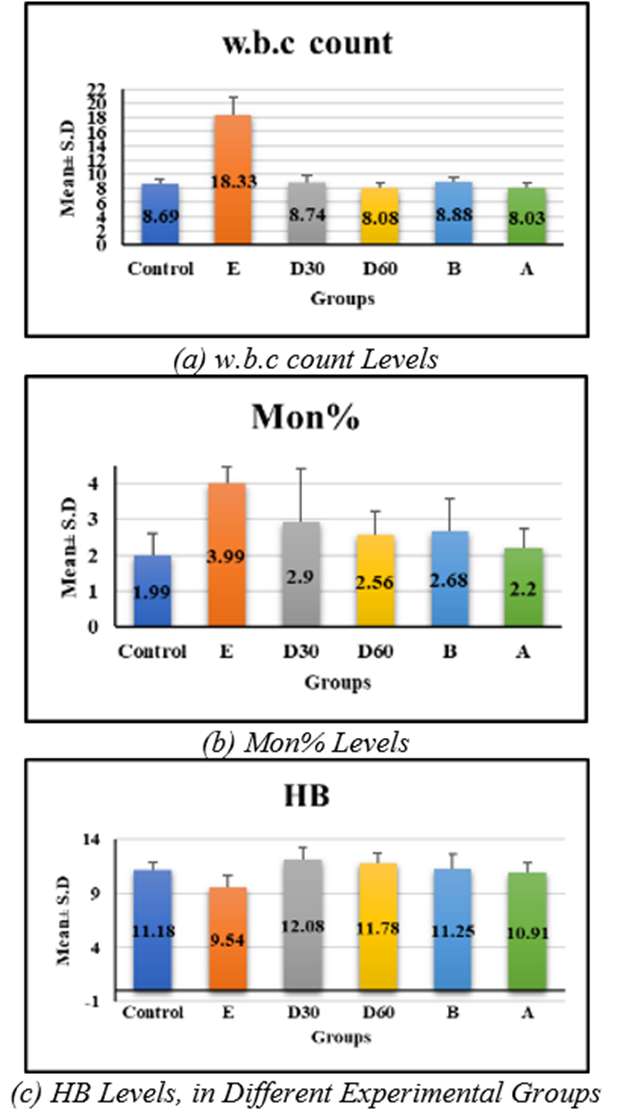
Fig. 1. Effect of Isoproterenol Injection and Losartan Treatment
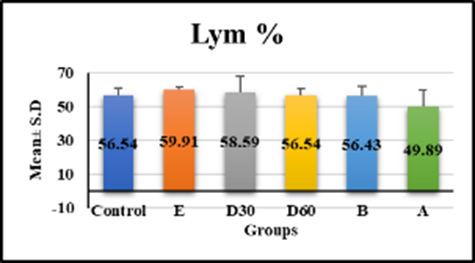
Fig. 2. Effect of Isoproterenol Injection and Losartan Treatment on Lym % Levels in Different Experimental Groups
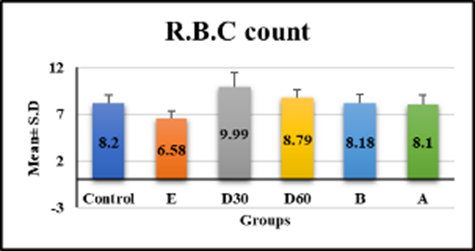
Fig. 3. Effect of Isoproterenol Injection and Losartan Treatment on R.B.C count Levels in Different Experimental Groups
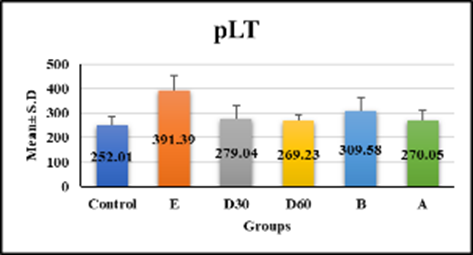
Fig. 4. Effect of Isoproterenol Injection and Losartan Treatment on pLT Levels in Different Experimental Groups
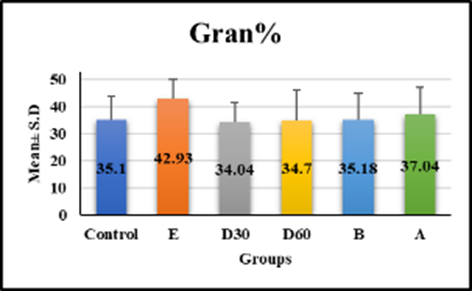
Fig. 5. Effect of Isoproterenol Injection and Losartan Treatment on Gran% Levels in Different Experimental Groups
Pearson correlation coefficient between study parameters in different experimental groups
The correlation matrix in (table 2) shows blood cell counts and other characteristics. W.B.C count's significant correlations (p < 0.01) include positive correlations with Lym, Mon, and Gran %, negative correlations with R.B.C count, HB, and pLT, and positive correlation with pLT (r = 0.673). Mon% is strongly correlated with pLT (r = 0.582) and moderately correlated with HB (−0.289). R.B.C count has a positive connection with HB (r = 0.368) and a negative association with pLT (r = -0.344) (p < 0.05). HB has a substantial negative correlation with pLT (r = -0.519). Lym %, Gran%, and Mon% also show varied associations.
Table 2
Pearson correlation coefficient between study parameters in different experimental groups
Parameters | w.b.c count | Lym % | Mon% | Gran% | R.B.C count | HB | pLT | |
w.b.c count | r | 1 | .264 | .514** | .283 | -.527** | -.613** | .673** |
P |
| .070 | <0.001 | .051 | <0.001 | <0.001 | <0.001 | |
Lym % | r |
| 1 | -.039 | .269 | .016 | .037 | .073 |
P |
|
| .794 | .064 | .916 | .803 | .622 | |
Mon% | r |
|
| 1 | -.095 | -.017 | -.289* | .582** |
P |
|
|
| .521 | .910 | .047 | <0.001 | |
Gran% | r |
|
|
| 1 | -.232 | -.256 | .222 |
P |
|
|
|
| .113 | .079 | .130 | |
R.B.C count | r |
|
|
|
| 1 | .368** | -.344* |
P |
|
|
|
|
| .010 | .017 | |
HB | r |
|
|
|
|
| 1 | -.519** |
P |
|
|
|
|
|
| <0.001 | |
pLT | r |
|
|
|
|
|
| 1 |
P |
|
|
|
|
|
|
| |
**. Correlation is significant at the 0.001 level (2-tailed).
r: Pearson correlation coefficient, P: p value
Immunohistochemistry result
The study found that while the control group had normal levels of mmp9 and TGF beta 1 protein expression, group E (heart infarction) had a significant increase in protein expression at 𝑃≤0.05 compared to the control group, while losartan treatment groups showed a significant decrease at 𝑃≤0.05 compared to group E (FIGURE 6A AND 6B) (fig. 1, 2, 3, 4). Losartan significantly reduced protein expression in induction group (E) due to isoprenaline injection, which produced myocardial infraction. Longer treatment with losartan may improve these results.
Immunohistochemical section
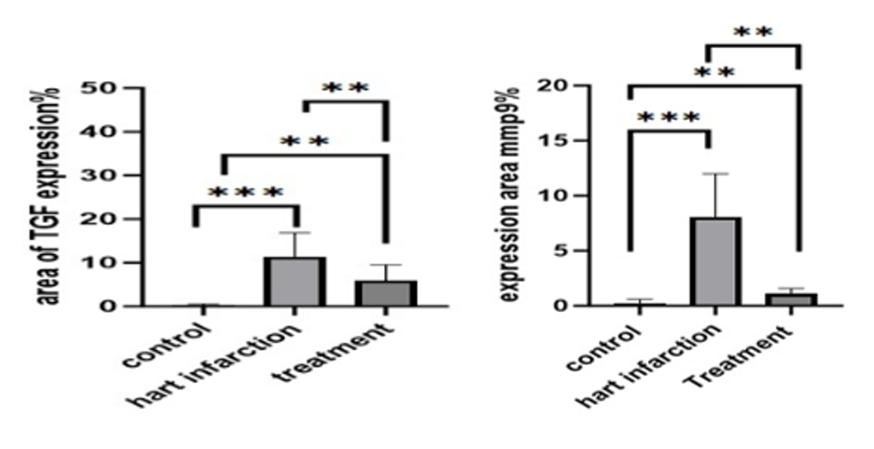
Fig. 6. Expression areas: (a) TGF bata 1 protein or marker in all experimental groups, at 𝑃≤0.05; (b) Expression areas of mmp9 protein or marker in all experimental groups, at 𝑃≤0.05
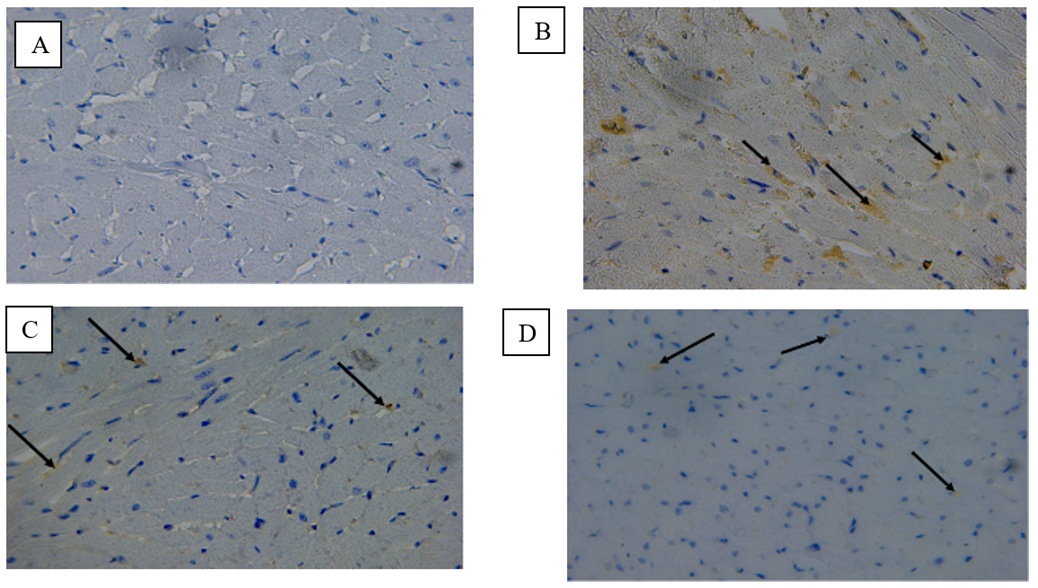
Fig. 7. A) Photomicrograph on rat heart from the control group (C) The expression of mmp9 in the myocardium showed negative immunoreactivity immunohistochemistry stained 40 X.B)Photomicrograph on rat heart isoprenaline injection group (E) The expression of mmp9 in the myocardium showed wide distribution of positive immunoreactivity in the myocardium (black arrows) immunohistochemistry stained 40 X.C)Photomicrograph on rat heart from losartan treatment from 30 days group (D30) The expression of mmp9 in the myocardium showed minimal positive immunoreactivity in myocardium fibers (black arrows) immunohistochemistry stained 40 X.D)Photomicrograph on rat heart from losartan treatment from 60 days group (D60) The expression of mmp9 in the myocardium showed minimal positive immunoreactivity in myocardium fibers (black arrows) immunohistochemistry stained 40 X
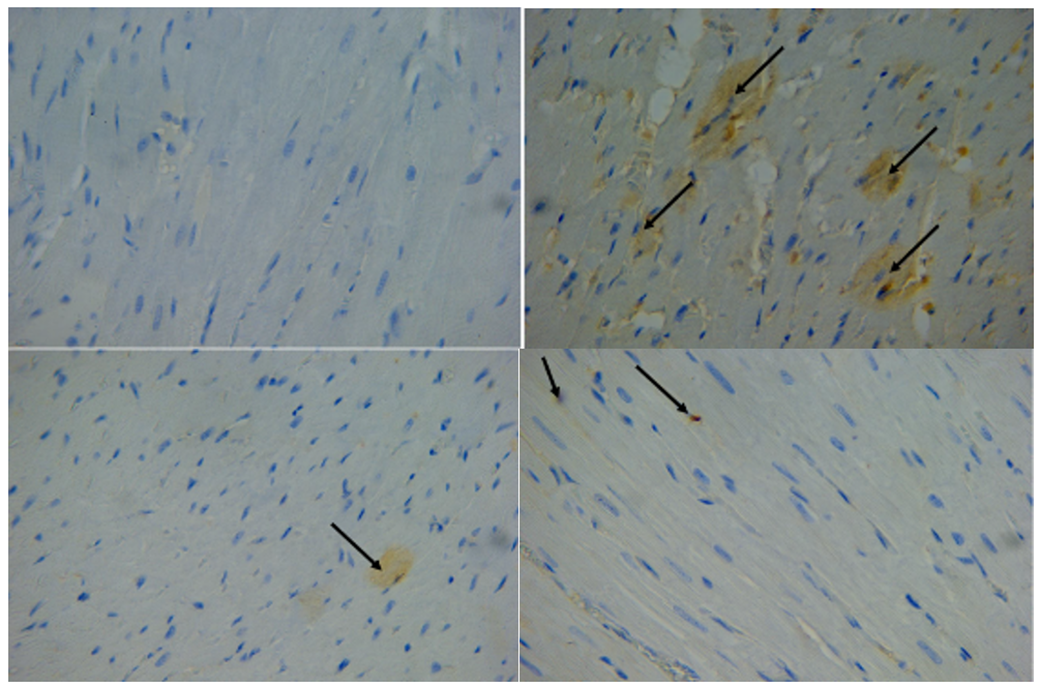
Fig. 8. A) Photomicrograph on rat heart from control group (c) expression the TGF-beta 1 in the myocardium showed negative immunoreactivity in myocardium fibers immunohistochemistry stained 40 X.B) Photomicrograph on rat heart from isoprenaline injection group (E) expression the TGF-beta 1 in the myocardium showed a wide distribution of positive immunoreactivity in myocardium fibers (black arrows); immunohistochemistry stained 40 X.C) Photomicrograph on rat heart from losartan treatment for 30 days group (D30) expression the TGF-beta 1 in the myocardium showed minimal positive immunoreactivity in myocardium fibers (black arrows); immunohistochemistry stained 40 X.D) Photomicrograph on rat heart from losartan treatment for 60 days group (D60) expression the TGF-beta 1 in the myocardium showed minimal positive immunoreactivity in myocardium fibers (black arrows); immunohistochemistry stained 40 X
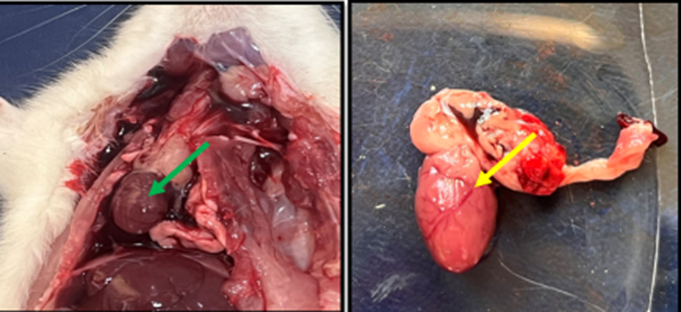
Fig. 9. Gross image of rat in control group showed normal size and shape of heart with normal color and surface (green arrow) while (yellow arrow) showed normal ventricle blood vessels
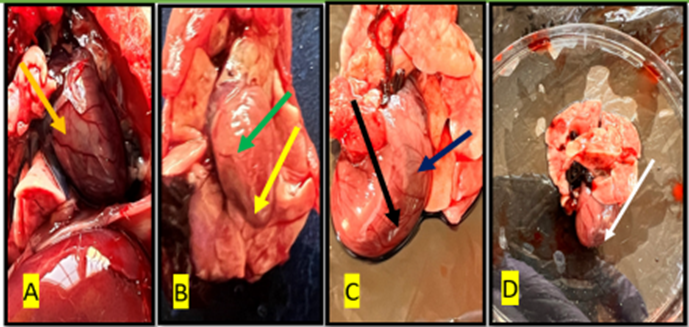
Fig. 10. Gross image of the heart of the rat from treated with isoprenaline (group E) The (white arrow) shows necrosis area in the ventricles, (black, blue, and red arrow) showed ventricular aneurysm, the green and yellow arrows show fluid accumulation(edema) and irregular surface
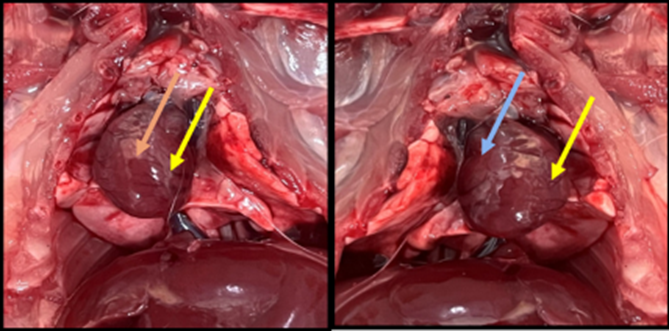
Fig. 11. Gross image of the rat’s heart in a group of losartan treatments for 30 days (D30). showed the normal volume and surface of the heart (blue arrows), show normal ventricles, blood vessels
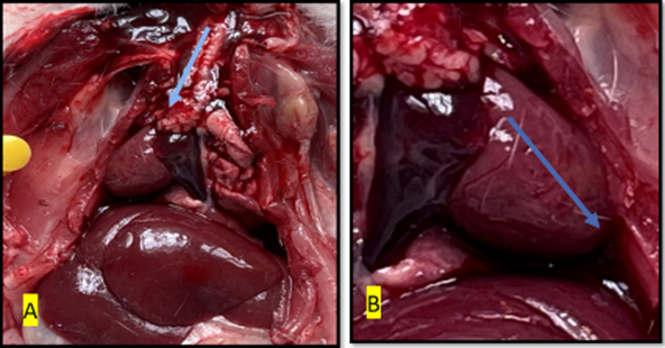
Fig. 12. Gross image of the rat’s heart in a group of losartan treatment for 60 days (D60) showed the normal volume, normal smooth surface of heart and normal ventricular blood vessels (blue arrows).
Microphotography Examinations
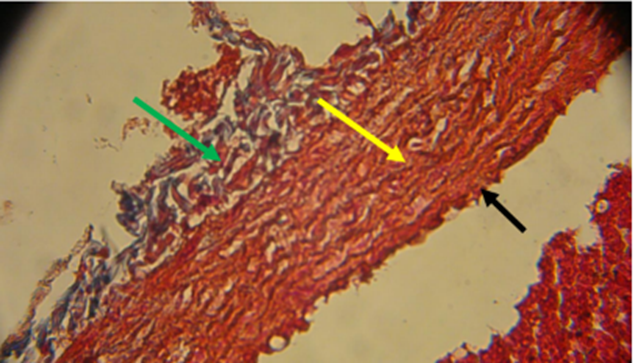
Fig. 13. Photomicrograph on rat heart from the control group showed normal blood vessel: tunica adventitia (green arrow) tunica media (black arrow) tunica intima (yellow arrow) stained with massone trichrome 40x
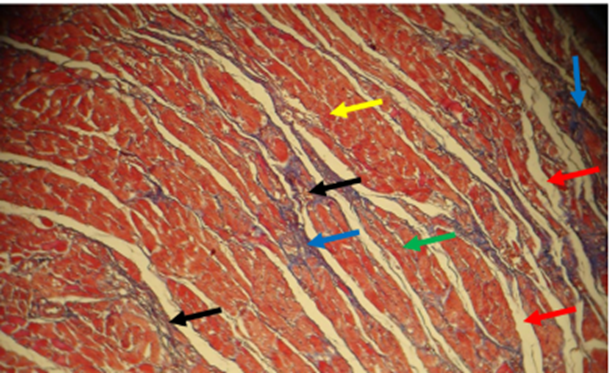
Fig. 14. Photomicrograph on rat heart from isoprenaline injection group (E) showed myonecrosis regions (black arrow) slender, atrophy cardiac muscles fibers (green arrow), the muscles fibers loss their organized and striation (yellow arrow), dilated spaces with strands of bundles separated the cardiac muscle fibers (red arrow) collagen fibers deposition beteen muscles fibers (blue arow) stained with massone trichrome 40 X
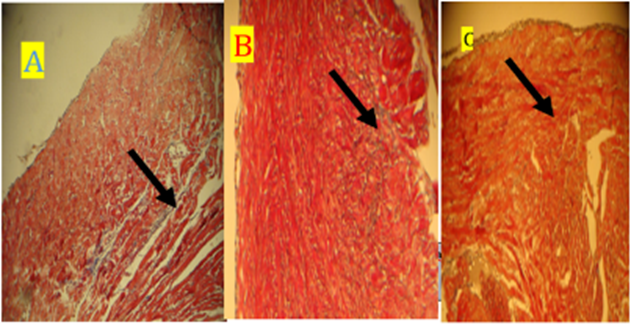
Fig. 15. Photomicrograph on rat heart (A) D30 Groupe and (B) D60 Groupe (C) Groupe B) losartan +isoperternol), all Groupe showed dcrease in collagen deposition (black arrow). Stained with masson trichrome (10X)
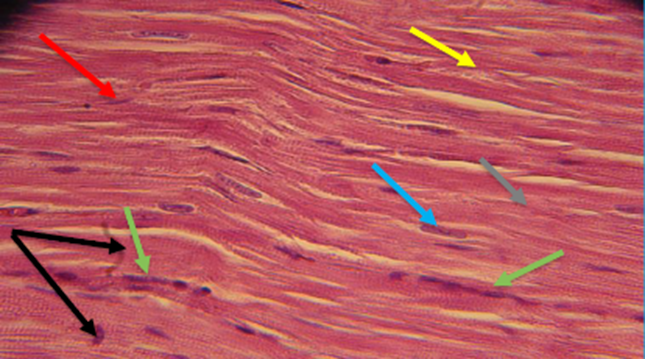
Fig. 16. The section of the control group rat heart showed normal branched cardiac muscles fibers organized longitudinally (gray arrow), centrally vesicular nuclei (blue arrow), striation of muscles fibers (black arrow), connective tissue separated the myofibers (yellow arrow), intercalated disc (red arrow), few number of fibroblast (green arrows), Stained with H&E (40 X)
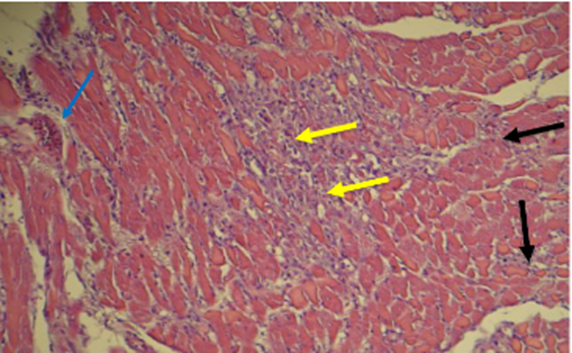
Fig. 17. Section of the heart of rat of isoproterenol injection Groupe (E) showed interstitial fibrosis (black arrow) inflammatory cells, fibroblast and heavy deposition of collagen fibers (yellow arrows) perivascular fibrosis (blue arrow) stained with H&E (10X -40 X)
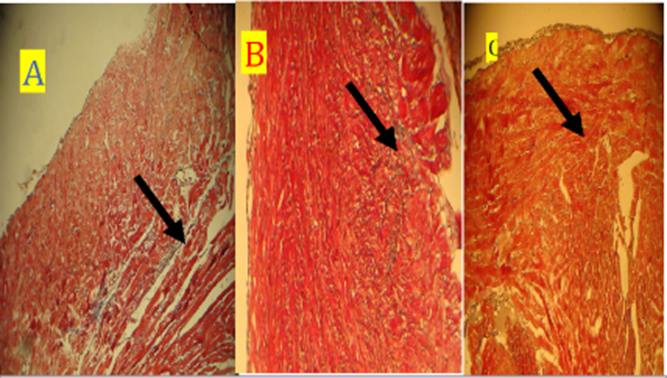
Fig. 18. Photomicrograph on rat heart (A) D30 Groupe and (B) D60 Groupe (C) Groupe B, all Groupe showed dcrease in collagen deposition (black arrow). stained with masson trichrome (10X)
Discussion
ISO, a β-adrenergic agonist, has been linked to infarct-like lesions in rats and other animals [14, p. 363-372]. Similar metabolic and structural abnormalities have been seen in experimental animals' heart tissue as in humans with myocardial infarction. Auto-oxidation of injected isoprenaline produces free radicals that peroxidise lipids, damaging the heart cell membrane. Release of proteolytic enzymes by inflammation is essential for heart tissue destruction [15, p. 2095-2128].
The rats given a dose of isoproterenol (85 mg/kg) in the current investigation died after the second dose in the experimental induction of myocardial infarction. These findings conflict with those published by [16, p. 285-293; 17, p. 1-8; 18, p. 529-535; 19, p. 36-42].
Myocardial infarction has also been observed as a side effect of large doses of isoprenaline [20, p. 450-464; 21, p. 316-330].
The rats in this study received an injection of 65 mg \kg.bw of isoprenaline for five days, and the blood samples showed positive troponin and dose-induced myocardial infarction outcomes.
Increased extracellular matrix production and increased expression of Transforming Growth Factor β (TGF-β) are associated with elevated blood glucose levels. The activation of RAAS is closely related to TGF-β. Through its stimulation of collagen synthesis and secretion mediated by angiotensin II, TGF-β plays a crucial role in cardiac fibrosis. The aetiology of fibrosis may involve the overexpression of TGF-β, which can lead to cardiac remodelling or hypertrophy and ultimately heart failure. The RAAS antagonists [22, p. 1006].
It is well known that ventricular remodelling and myocardial fibrosis are important pathophysiological variables that worsen outcomes following myocardial infarction. Ventricular remodelling and cardiac fibrosis are strongly linked to the TGF-β1/Smad signalling pathway. Several studies have shown that TGF-β1/Smad signalling has a critical role in controlling the synthesis of matrix metalloproteinases (MMPs) and promoting the activation of the renin-angiotensin-aldosterone system (RAAS). Through its interaction with the plasma membrane's TGF-β1 receptor, it encourages Smad2/3 to become phosphorylated, which leads to their connection with Smad4 to form a complex that translocates into the nucleus and initiates gene transcription. TGF-β1 is significantly upregulated in the myocardium after myocardial infarction (MI), according to several experimental studies [23, p. 832-838; 24, p. 153338; 25, p. 1605].
Immunohistochemistry staining image analysis results revealed that the control group's mmp9 and TGF-beta 1 protein expression area was within a normal range, while group E's (heart infarction) protein expression area showed a significant increase at 𝑃≤0.05 when compared to the control group, and a significant decrease at 𝑃≤0.05 when compared to group E.
These findings demonstrated that losartan significantly reduced the region of protein expression that rose in the induction group (E) as a result of the isoprenaline injection, which resulted in myocardial infraction; these outcomes might get better with longer-term losartan treatment. This outcome is consistent [26, p. 607].
However, the research of Zahra [27] Hypertension is one risk factor for cardiovascular disease that affects people everywhere. Rates of prescriptions for the antihypertensive drug "sartan" The main function of losartan, candesartan, and valsartan is to block the angiotensin receptor. It functions as an inhibitor of urate transporters. For individuals who are intolerant to angiotensin-converting enzyme inhibitors, it is beneficial as a second-line treatment for systolic dysfunction, myocardial infarction, congestive heart failure, coronary artery disease, and other relevant medical conditions. respiratory conditions, high blood pressure.
As it has been proposed that losartan may have potential for the treatment of knee joint fibrosis in OA, numerous studies have demonstrated its therapeutic efficacy against a variety of disorders. This finding might be easily translated into the clinical environment [28, p. 114121].
Triamcinolone (F-TA) and losartan-loaded in situ forming gel (F-LG) have similar effects in terms of improving vascularity and pigmentation. F-LG performed better than triamcinolone in terms of fibrosis and collagen density reduction, and it had a greater effect on improving hypertrophic scars.
Although triamcinolone is currently considered the gold standard for treating hypertrophic scars, this study showed that losartan-loaded in situ forming gel could be a good substitute, outperforming triamcinolone in many cases in terms of the appearance and pathological features of hypertrophic scars. It can also be used to treat several hypertrophic scars in combination with triamcinolone [29, p. 1-11].
Losartan may minimise the risk of kidney injury by inhibiting the increased signalling of Ang II/AT1/TGFβ1, which may avoid neurogenic paralysis bladder NPB fibrosis [30, p. 137-146].
Also, the present showed the hematological parameters, a significant increase at (P˂0.001) in w.b.c count in group E (heart infraction induced) in comparison with control and a significant decrease at (P˂0.001) in the losartan treatment group and significant decrease at (P< 0.001) in losartan treatment groups in comparison with group E, no significant difference at (P< 0.001) in losartan treatment group in compression with control, also the present study showed the Differential WBCs count, the level of lymphocyte significant increase at (P< 0.05) in group E in comparison with control and significant decrease at (P< 0.05) in all losartan treatment group in comparison with group E, the monocyte and granulocyte level showed significant increase in group E, at (P< 0.001) in comparison with control, while significant decrease at (P< 0.001) in all losartan treatment groups in comparison with group E, no significant difference between losartan treatment groups and control group, moreover the recent study showed the level of red blood cells significant decrease at (P< 0.001) in group E in comparison with control and significant decrease at (P< 0.001) in all losartan treatment groups in compression with group E and no significant difference at (P< 0.001) in comparison with control group, also the level of Hb has significant decrease at (P< 0.001) in group E comparison with control group, and significant increase at (P< 0.001) in all losartan treatment groups in comparison with group E and no significant difference at (P< 0.001) in comparison with control finally the level of platelets significant increase in group E in compression with control and significant decrease at (P< 0.001) in all losartan treatment group in comparison with group E, the current study showed that the result in the infarction group (E) indicated that isoproterenol injection leads to an increase in the number of white blood cells due to the inflammation in the myocardium and infiltration of inflammatory cells, and causes severe anemia, as we noticed that the animals injected with isoproterenol suffered from strange movement inside the cages and decrease food intake with head raised up, also isoproterenol injection lead to increase in blood platelets due to inflammation and fibrosis in myocardium and around the blood vessels. Losartan improved w.b.c, RB.C, Hb, and platelet levels to normal levels in the control group, indicating an anti-inflammatory action.
In this study, isoproterenol injection to induce myocardial infraction caused irregular, atrophied, and degeneration of myocardial muscle fibres with wide space between them, myonecrosis, nucleus degeneration and dislocation, cytoplasm vacuolation, and increased inflammatory cell and fibroblast infiltration. The study found collagen fibres and adipose tissues between cardiac muscle fibres, oedema in myocardium, fibrosis around blood vessels, dilatation of blood vessels with amyloid fluid, thrombosis, hemolyzed red blood cells, and hyperplasia of blood vessel layers, consistent with findings from previous studies [31, p. 175-185; 32; 33; 34, p. 14-35]. Congested, dilated blood capillaries with blood extravasation, inflammatory cell infiltration, and exudates among malformed heart muscle fibres. Deteriorating cardiac myocytes featured black pyknotic nuclei and acidophilic sarcoplasm.
Also in the current study the treatment with losartan for 30 days showed improvement to histopathological alteration such as showed the cardiac muscles fibers regularly organized and form bundles with mild connective tissues separated between them, and most of the nucleus is central with mild infiltration of inflammatory cells, the blood vessels still congested with wavey cardiac muscles fibers around it, and normal ventricular wall: epicardium, myocardium and endocardium, while in treatment with losartan for 60 days the result have improved even more, so that a normal ventricular wall, epicardium, myocardium and endocardium; the muscle's cardiac fibers appeared regularly organized with obvious striation, central nucleus and intercalated discs are junctions between cardiac muscle fibers, reduced inflammation and inflammatory cells, with a decrease of collagen fiber disposition between cardiac muscles fibers and few of fibroblast cells, where the histological results were closer to the normal tissue in the control group in the group B (isoproterenol injection + losartan treatment) The study showed histological changes in group B (losartan medication for 55 days, then isoprenaline injection for 5 days, losartan's protective effect). Longitudinal and transverse cardiac muscle fibres with central nuclei, minor oedema and congestion, fewer gaps between fibres, mild interstitial fibrosis, and adipose tissue deposition.
Histological findings improved dramatically in this group. The current study revealed that losartan is crucial in treating induced myocardial infarction, and the longer treatment duration enhanced group B's result. The current study found that losartan protects against and lung heart mass index in both ventricles, regardless of rat strain. induced myocardial infarction, supporting Hyun Soo Park [35, p. 573-581]. This study found that ramipril and a defined average dose of losartan control blood pressure more efficiently than either medicine alone in hypertension and may protect the heart and blood vessels from atherosclerosis and myocardial infarction [36, p. 465-470]. This study showed Both drugs reduced BNP-45, ANP, and TBARS in both heart ventricles.
In our DOXO-induced chronic cardiotoxicity model, losartan improved diastolic dysfunction, SERCA2a level, and tissue inflammation but not systolic dysfunction [36, p. 465-470; 37], losartan reduces ventricular remodelling in myocardial infarction, improving survival, ventricular hypertrophy and dilation, and isovolumetric pressure. Also, this treatment does not change myocardial collagen concentration.
The best uric acid-lowering drug for HF patients was losartan and dapagliflozin [38; 39, p. 393-402], apelin +losartan reduced infarct size by 30, 33, and 48%, respectively, and improved left ventricular function parameters like developed pressure.
The histopathological section stained with Masson trichrome showed heavy deposition of collagen fibres between cardiac muscle fibres, vacuolization of sarcoplasm, and deposition of adipose tissue. The muscle fibres lost their organisation and striation with dilated spaces and strands of bundles separated.
Also revealed plexiform, perivascular, and thick collagen fibres around blood vessels.
This study found that losartan improved results in all treatment groups and reduced collagen fibre deposition in cardiac muscle fibres and around blood vessels, suggesting that it may work as an anti-fibrosis treatment [40, p. 2115-2134; 41, p. 815-825].
In the histopathological section stained with Masson trichrome, the present study showed histopathological section from isoproterenol injection group E (heart infraction group) heavy deposition of collagen fibers between cardiac muscles fibers, vacuolization of sarcoplasm, and deposition of adipose tissue also showed slender, atrophy cardiac muscle fibers, and the muscle fibers lost their organisation and striation with dilated spaces with strands of bundles separating the cardiac muscle fibers and heavy collagen fibers deposition between muscle fibers. Also showed plexiform fibrosis, perivascular fibrosis, dense collagen fibers around the blood vessels.
Conclusion
The significant finding of the present study suggests comparatively low and non-lethal doses of Isoprenaline can induce severe myocardial necrosis. And find that losartan has an effect in the treatment of myocardial infarction induced by isoproterenol in rats. Also find that the administration of losartan has significantly change hematological examination and minimize the histological lesions. Finally, the current study find that the duration of losartan use as a treatment for myocardial infarction has an impact on its effectiveness, where the longer the treatment, the more improved the drug's efficacy becomes.
.png&w=384&q=75)
.png&w=640&q=75)