Introduction
Four-coordinated copper(+2) chelate compounds formed by bi- and tetradentate organic ligands exhibit high catalytic activity in the reactions of the liquid-phase oxidation of organic substrates with molecular oxygen [1]. The catalytic properties of these compounds significantly depend on the spatial structure of their chelate nods [2,3]. In general case, four-coordinated copper(+2) chelates form square-plane or tetrahedrically-disrorted chelate nods.
Tetrahedrically-coordinated copper(+2) compounds have been established to show the highest catalytic activity in oxidation reactions by molecular oxygen among chelate complexes of different spatial structure of chelate nods. A well-known example is the indifferent behavior of octahedrically coordinated copper(+2) ions, e.g., [Cu(Н2О)6]2+ in Н2О2 solutions and tetrahedric copper(+2) ions [Cu(NН3)4]2+, in the presence of which Н2О2 decomposes with an explosion. A similar property is exhibited by polydentate chelate copper(+2) complexes. However, the spatial structure of chelate nod is not the only property to affect catalytic activity of the copper(+2) chelates. The chemical nature of coordinated ligand atoms, donor-acceptor properties of distant substituents, presence or absence of the conjugation in metal chelate quasiaromatic cycle and its size also influence on catalytic properties of the copper(+2) chelate complexes [2-4].
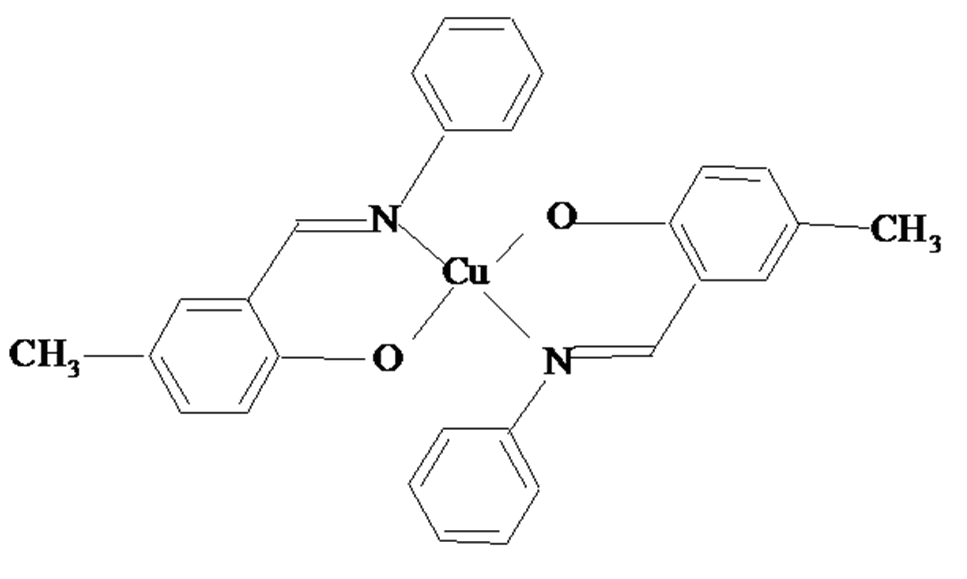
Fig. 1. Structure of copper(2+) chelate compound
The aim of this work was to find a correlation between catalytic and antifungal activities of bicyclic copper(+2) chelate compound of Shiff base derived from salicylic aldehyde and 1-aminoethyl benzene (Fig. 1). The oxidation of benzildihydrazone by molecular oxygen was studied as model reaction.
Experimental
Copper(+2) chelate compound was synthesized by the reaction of as-perchased Cu(CH3COO)2·H2O with the ligand HL under reflux in ethanol. The ligand HL (Fig. 1) was obtained by the reaction of (1-aminoethyl)benzene with m-methyl salicylic aldehyde. The chelate compound CuL2 was characterized with elemental analysis data, IR-spectroscopy and electronic spectra in toluene. Toluene was preliminary distilled.
The oxidation of the substrate was carried out by oxygen of the air (20.8 kPa) in a solution of toluene under intensive stirring with magnetic bar at 7.5 Hz. Benzildihydrazone was twice recrystallized from toluene. Thin layer chromatography was used for characterization of reagents and products.
Results and discussion
The structure of chelate compound CuL2 is presented in Fig. 1. Deprotonated bidentate ligand HL forms uncharged copper(+2) chelate complex with tetrahedrically distorted chelate nod of coordinated O and N atoms of the ligand.
Benzildihydrazone and its toluene solutions are stable in air. However, this compound is known to be oxidized by oxygen under heat treatment in the presence of some solids, e.g. Cu2Cl2 or Cu(OAc)2 to form diphenyl acetylene (tolan) in accordance with the reaction 1 [5].
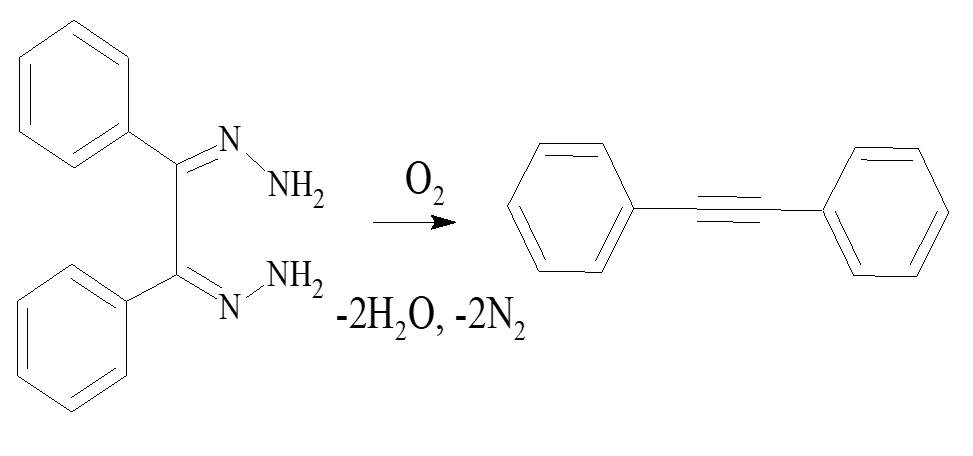
Metal chelate compounds are usually more effective as homogeneous catalysts in a liquid phase. We found that mixing of toluene solutions of benzyldihydrazone in the absence of complex compound CuL2 does not lead to a change in a volume of the gas phase and the composition of the solution. The change in the volume of the gas phase occurs only in the presence of the homogeneous catalyst CuL2 when stirring with a magnetic bar.
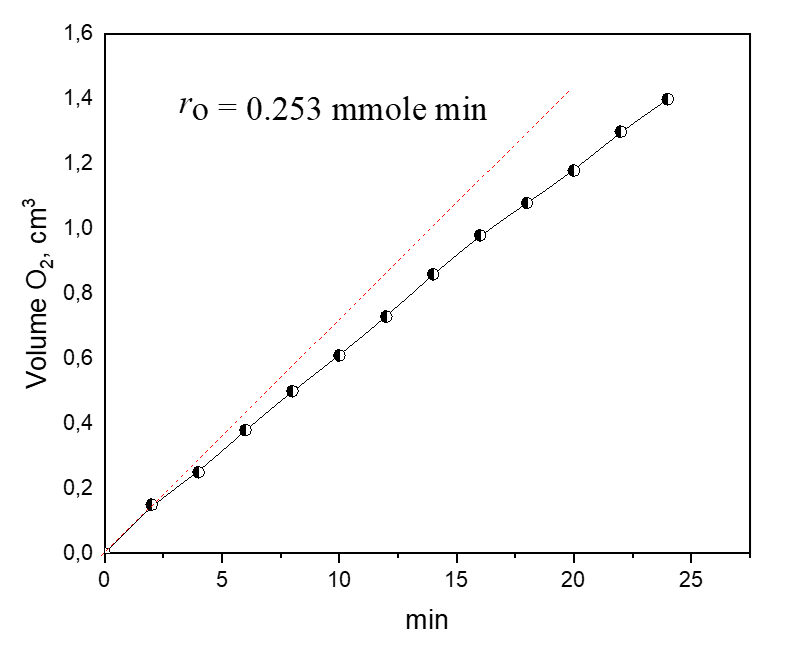
Fig. 2. Liquid-phase oxidation of benzildihydrazone (initial C1 = 0.0315 mole l-1) in toluene by molecular oxygen at 298 K in the presence of 2.92×10-3 mole l-1 of CuL2
The kinetics of benzildihydrazone oxidation by molecular oxygen was studied by measuring the initial rates via graphical differentiation of kinetic curves. As an example, figure 2 shows the kinetic curve of liquid-phase oxidation of benzyldihydrazone by molecular oxygen catalyzed by chelate compound CuL2 as a function of the volume of oxygen found by the change in the volume of the gas phase.
In accordance with the basic postulate of chemical kinetics, the current rate of a homogeneous catalytic process can be represented as equation 1:
r = kef CCu(C1)n(C2)m, (1)
where kef is effective rate constant, Ccu is concentration of the catalyst, C1 and C2 are curent concentrations of benzildihydrazone and oxygen, respectively.
A rate of the reaction catalyzed by homogeneous chelate catalyst is proportional to a catalyst concentration (n = 1) which is reasonably constant during the reaction. Provided that the initial concentration of oxygen (C2)0 is also constant at a constant temperature, equation 1 can be represented as follows:
ln[r0 /Ccu] = ln[kef×(C2)0m] + nln[(C1)0] (2)
where r0 is an initial rate of the reaction 1 and (C2)0 is initial concentration of oxygen.
Initial concentration of oxygen was calculated according to Dalton equation: Po = Σpi, where Po is atmospheric pressure and pi are partial pressures of O2, N2, and toluene. As the initial concentration of oxygen is constant in closed system at constant temperature, equation 2 represents a linear function of ln[r0/CCu] against ln[(C1)0].
Independent experiments were carried out under random conditions, i.e., at different concentrations of the catalyst and at different initial concentrations of the substrate. The initial rates of the benzildihydrazone oxidation were calculated by graphical differentiation of the kinetic curves at t = 0. The results obtained are listed in Table.
Table
|
|
(C1)0, mole l-1 |
CCu, mmole l-1 |
r0, cm3 l-1 min-1 |
|
1 |
0.042 |
2.92 |
7.30 |
|
2 |
0.0315 |
2.92 |
6.10 |
|
3 |
0.021 |
2.26 |
3.03 |
|
4 |
0.010 |
3.33 |
2.30 |
The data were depicted in Figure 3 as a function of ln[r0 /Ccu] against ln[(C1)0]. As can be seen, this function is linearly dependent on ln[(C1)0]. The slope of this dependence n ≈ 1.
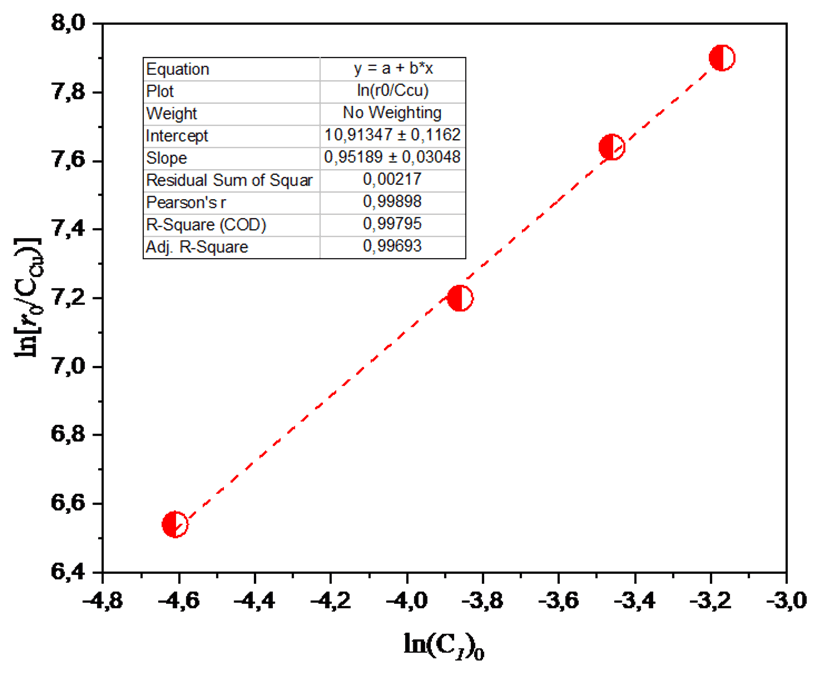
Fig. 3. Plot of ln[r0/CCu] as a function of ln[(C1)0]
The copper(2+) chelate compound CuL2 was also established to exhibit high antifungal activity against bread mold (Mucor mucedo). As can be seen from Fig. 4, the surfaces of the four central samples untreated with catalysts are compleetly affected by bread mould. The right halfs of the upper and lower samples were treated with 0.2 and 0.1 ml, respetively, of 1´10-4 mol l-1 toluene solutions of CuL2. After the removal of toluene, dried samples were exposed in water-saturated chamber for 5 days at 298 K.

Fig. 4. Antifungal activity of CuL2 against bread mold (Mucor mucedo) after exposure in a chamber with saturated water vapor for 5 days at 298 K. Four central samples were untreated with catalysts
It can be seen that only the left sides of the samples, which were untreated with catalyst, are affected by bread mold. This data indicates that CuL2 high catalytic activity to liquid-phase oxidation of benzildihydrazon with molecular oxygen also effectively inhibits the development of the bread mold demonstrating high antifungal activity.

.png&w=640&q=75)