1. Introduction
Fruit juices are beverages containing nutrients, vitamins and minerals, which are essential for health. Fruit juices can be a potential source of toxic elements, some of them having a cumulative effect or leading to nutritional problems due to low or high concentration of essential elements [1, p. 871–878]. Thus, in order to guarantee food safety, fruit juices require careful investigation. Owing to heavy metal contamination of the environment, the analysis of trace elements in seasonal fruit samples as well as in their products has gained considerable attention [2, p.45–50]. Several analytical techniques have been proposed for detection of metals ions in fruit juices AES [3, p.619–622], the mass spectrometry with the limit of determination of 0.1 µg*L−1. The technique of chronopotentiometry [4, p.11–53] and polarography [5, p. 28–34] were found quite applicable and cost-effective for determination of metals ions can be used for effective evaluation of beverages quality in the range of 10–180 µg*L−1. These techniques of metals ions determination, including atomic absorption spectroscopy [6, p. 28–34], inductively coupled plasma spectrometry and electrochemistry often imply serious limitations for on-site determination of metals ions in environmental samples. Among the available techniques of metals ions detection colorimetric optodes are of particular interest. This sensing approach clearly is promising one as to development of commercial indicators, such as test strips that can be assessed visually. Such optical sensors are a promising line of research in development of measurement and monitoring methods for various objects. Metal ions concentrates into the sensor bulk and them content is assessed according to the spectral characteristics in the visible spectrum. Analysis can be carried out with the use of optical sensors based on a transparent polymer matrix (TPP) modified by a hydrophilic component such as polyethylene glycol (PEG). The structure of TPP improves sorption of the organic compound into the body of the matrix and helps increase sensitivity of this analytical method [7, p.117-119]. The main purpose of this study was to determine heavy metals ions sum (MIS) concentrations in fruit juices. The combination of solid-phase extraction and naked-eye determination of metals ions allows creating a simple and sensitive colorimetric sensor based on the TPP. Introduction of 1-(2-pyridylazo)-2-naphthol (PAN) or 4-(2- pyridylazo)-resorcin (PAR) into the sensor provides the color reaction with metals ions [8, p.1475–1479].
2. Experimental
2.1. Polymethylmethacrylate matrix preparation
TPP is a specially created material containing functional groups, which make it possible to extract both organic reagents and metal ions. Transparent 10×10 cm polymethacrylate plates 0.60±0.04 mm thick were prepared by radical block polymerization of methacrylate with 5 % PEG 400 and (alkyl)acrylates of alkaline metals at temperature 60–70 °C for 3–4 hours. These plates were then diced into 6.0 × 8.0 mm working plates weighing about 0.05 g each.
Immobilization into TPP was performed by their sorption from water-alcohol (25 %) of 2.5•10-4 M PAN or 5.0•10-4 M PAR solutions for 5 minutes. The absorption spectra of PAN or PAR in the polymethacrylate matrix corresponds to these spectra in chloroform. As a result, we obtained a sensing element colored yellow for quantification metals ions with absorption peak at (525*15) nm.
2.2. Sum of metals ions determination
Commercial fruit juices from the most consumed brands were purchased in Tomsk, between March and June 2018. The juice fruits samples comprised juices from peach, multi-fruit, orange, apple, mango and pineapple. The 50 ml fruit juice was placed in a 200 mL flask, then 20 mL of 15 % HNO was added and the mixture was diluted with distilled water to the mark. Next, we dipped the TPP plates into flask and mixed the contents with a mechanical mixer for 20 minutes. The plates were then taken out and dried between sheets of filter paper. The absorption was measured at 520–530 nm. The concentration of metals ions was determined according to the spectrophotometric calibration dependence constructed in the concentration range from 20 to 3600 µg•L-1. The color scale for visual determination was constructed in the concentration range of 0–1000 µg•L-1.
2.3. Apparatus and reagents
The absorption spectra of TPP and of the solutions were recorded on Evolution 2011 spectrophotometer (Thermo Fisher Scientific Inc., USA) against a blank polymer plate prepared under the same conditions. The pH values were measured by I-160 ionometer (Izmeritelnaya Tekhnika NPO, Russia) with a pH-selective glass electrode. The ionometer had an absolute error of ± 0.020 рН and was calibrated at 25 °С using buffer solutions with pH 1.00 and 9.18. The resulting solutions were stirred for 5–30 minutes in a Multi Bio RS-24 multirotator.The stock solution of 5.0 mg·L−1 of metals ions (Fe, Cd, Co, Zn, Pb, Ni, Cu and Mn in equal parts) was prepared by dissolving MeCl in water and diluting it in a volumetric flask with distilled water to 100 mL. The 0.01–1.0 mg·L−1 working solutions were prepared by dilution. The desired pH level was obtained using HNO3, chemically pure and analytical grade reagents were used.
3. Results and discussion
PAN has been long since recognized as a sensitive colorimetric reagent for heavy metals ions, forming intensely colored stable complexes due to the presence of a thiol group (Safari et al., 2011) in it. In the presence of metals ions and depending on its concentration, the color of the optode changes to purple because of the formation of purple complex. This allowed a direct accurate measurement of optical and visual (Fig. 1) characteristics of the optode.
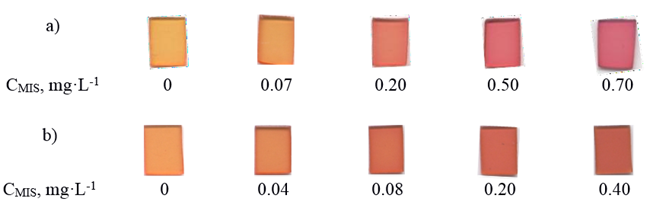

Fig. 1. The scanned images of TPP modified with PAN or PAR after contact with MIS solution of various concentrations, V = 50 mL, pH 5.2
Extraction of metals ions and its reaction with PAN in the body of TPP are irreversible. This creates some problems, for example, TPP cannot be used for analytical methods involving desorption of a target substance. The colorimetric change upon metals ions binding in the optode presented here could be used in potential portable devices for naked-eye detection because color intensity of TPP is inversely proportional to metals ions concentration. It can be observed that the color intensity is evenly distributed along the detection area. Dependences of the analytical signal on pH of the solution and on the contact time were studied to establish the optimal conditions for MIS determination. The dependence of the optical density of TPP modified PAN on the pH of metals ions water solution is shown in Fig. 2.
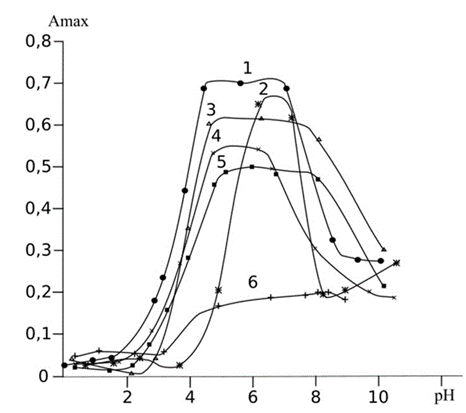
Fig. 2 Optical density of TPP modified with PAN depends on pH of metals ions water solution Ni2+ (1), Mn2+ (2), Zn2+ (3), Cu2+ (4), Cd2+ (5), Pb2+ (6) (С Metal ions=20 mg•L-1, V=50 mL, t=10 min)
The optimum pH of PAN and metals ions complex obtained was pH 5.2. At pH 3–4, the reaction between PAN and metals ions was unstable due to protonation of sulfur atom which reduced the donor-acceptor interaction. Whereas at pH 6–9, the reaction was unstable because metals ions ion formed hydroxyl complex and precipitate. Accuracy of the proposed method has been validated using the reference method (Table 1, 2). The detection limit of 20 µg•L-1 was calculated for spectrophotometric method at a signal-to-noise ratio 3.
Table 1
Parameters of the analytical characteristics for determination of metals ions in fruits juices
| Sample | Proposed method | Reference method | ||
| Found | Sr | Found | Sr | |
| Peach 1 | 0.27 ± 0.06 | 0.18 | 0.28 ± 0.03 | 0.09 |
| Peach 2 | 0.23 ± 0.05 | 0.18 | 0.23 ± 0.03 | 0.10 |
| Peach 3 | 0.19 ± 0.04 | 0.17 | 0.19 ± 0.02 | 0.08 |
| Multi-fruit 1 | 2.75 ± 0.22 | 0.06 | 2.75 ± 0.30 | 0.09 |
| Multi-fruit 2 | 0.95 ± 0.11 | 0.09 | 0.96 ± 0.05 | 0.04 |
| Multi-fruit 3 | 0.59 ± 0.07 | 0.10 | 0.60 ± 0.04 | 0.05 |
| Multi-fruit 4 | 1.11 ± 0.12 | 0.09 | 1.11 ± 0.08 | 0.06 |
| Orange 1 | 0.37 ± 0.06 | 0.13 | 0.37 ± 0.04 | 0.09 |
| Orange 2 | 0.14 ± 0.02 | 0.12 | 0.16 ± 0.02 | 0.10 |
| Orange 3 | 0.11 ± 0.02 | 0.12 | 0.10 ± 0.01 | 0.08 |
| Apple 1 | 0.14 ± 0.02 | 0.12 | 0.14 ± 0.02 | 0.12 |
| Apple 2 | 0.22 ± 0.05 | 0.18 | 0.21 ± 0.03 | 0.12 |
| Apple 3 | 0.25 ± 0.05 | 0.16 | 0.25 ± 0.03 | 0.10 |
| Mango 1 | 0.49 ± 0.08 | 0.13 | 0.50 ± 0.06 | 0.10 |
| Mango 2 | 0.62 ± 0.08 | 0.10 | 0.63 ± 0.06 | 0.08 |
| Pineapple 1 | 1.37 ± 0.27 | 0.20 | 1.38 ± 0.09 | 0.05 |
| Pineapple 2 | 1.25 ± 0.21 | 0.13 | 1.25 ± 0.09 | 0.06 |
| Pineapple 3 | 0.97 ± 0.15 | 0.12 | 1.00 ± 0.08 | 0.06 |
Table 2
Metal ions levels (mg·L−1) in different fruit juices (n=5)
Detection method | Linear range, mg*L-1 | Regression equation | LOD , mg*L-1 |
| Visual with TPP (PAN) | 0.04-0.7 | A=0,34Cmetal ions+0.50 (R=0,9950) | 20 |
| Spectrophotometry with TPP | 0.01-3000 | A=0,14Cmetal ions + 0.07 (R=0,9904) | 3 |
| Reference method | 0.002-2100 | 0,2 |
Enrichment factor was calculated as the ratio of the calibration graph slopes with and without preconcentration of MIS and was found to be 37. The obtained results show that the proposed method is suitable for determination of MIS in such samples for the entire range of the concentrations studied. The optode demonstrated chromogenic behavior pertaining to metals ions as evidenced by noticeable color changes of the solutions from yellow to purple, which offers a potential for development of portable devices for naked-eye detection.
Conclusion
In this work a novel and sensitive analytical sensor for metals ions preconcentration and determination in fruit juices by solid phase extraction into TPP with immobilized PAN has been presented. The proposed method requires only a TPP plate as an extraction unit. Linearity was found at concentration ranged from 20 to 360 µg·L-1 with the R value exceeding 0.999. The proposed method has been successfully applied to visual and spectrophotometric determination of metals ions in fruit juices.

.png&w=640&q=75)