1. Introduction
Algae are photosynthetic organisms that live in water and have chlorophyll as their main pigment. They are devoid of roots, stems, or genuine leaves and possess uncomplicated reproductive processes lacking a protective covering of non-reproductive cells. Algae are categorized according to their nucleus type, where blue-green algae are classified as prokaryotic and all other types of algae are classified as eukaryotic [1, p. 15-19]. Algae can be categorized into two groups: macroalgae, which includes seaweeds, and microalgae, which encompasses green algae, blue-green algae, yellow-green algae, golden algae, and diatoms [2, p. 97]. Microalgae are single-celled organisms that carry out photosynthesis and occasionally engage in heterotrophy, contributing around 60% of the oxygen on Earth. They can serve as a source of nourishment, nutritional enhancements, and have several industrial uses [3, p. 591-600]. Algae are abundant in freshwater environments and play a crucial role as primary producers. Nevertheless, their abundance can result in detrimental effects such as the formation of algal blooms, which can deplete oxygen levels and cause environmental issues. Additionally, they can produce poisons that are toxic to all forms of life [4, p. 169-184]. Numerous studies have specifically examined epiphytic algae on aquatic plants because of their significant contribution to primary productivity. Epiphytic algae are the most prevalent organisms in aquatic systems and have a vital function in preserving ecological equilibrium [5, p. 252-259]. Epiphytic algae abundance is affected by variables such as fertilizer availability, light intensity, and water movement. Controlling these parameters can effectively manage the growth of epiphytic algae and ensure a harmonious equilibrium between algae and aquatic plants in environments.
Marine algae are intriguing because they exhibit a diverse array of biological activities, such as antibacterial, antiviral, antifungal, anti-allergic, and anticoagulant capabilities [6, p. 1187-1209; 7, p. 2448; 8]. Performed an ecological investigation on the abundance and characteristics of epiphytic algae on Ceratophyllum demersum and Phragmites australis in the Tigris River located in Baghdad [9, p. 37-52]. Discovered a total of 209 species of epiphytic algae on Ceratophyllum demersum and Phragmites australis. The study also observed that certain types of algae were specifically adhered to particular areas of the plants [10]. Carried out a systematic examination of epiphytic algae on different aquatic plants in the Hawizeh Marsh and discovered multiple previously unidentified species in Iraq.
2. The Study Area
The Euphrates River starts in Turkey and flows through Syria and Iraq. It crosses into Iraq through the Al-Bukamal region. The Euphrates River in Turkey receives a substantial amount of water from various important tributaries, which make up about 88.70% of the river's flow. In Syria, the remaining 11.30% of the river's water comes from other tributaries. There are no rivers that flow into the Euphrates River inside the borders of Iraq. The river has a total length of 2,290 km, with 40.8% of its course located in Turkey, 23.7% in Syria, and 35.4% in Iraq. The Euphrates River holds the distinction of being the longest river in the Middle East, boasting an average annual discharge of over 30 billion cubic meters [11, p. 1-12]. After crossing into Iraq, the Euphrates River creates a delta area between Hit and Ramadi, which stretches for 150 km until it reaches the Hindiya Barrage. At this point, the river divides into two branches known as the Hilla River and the Hindiya River [12]. Four sites were chosen to collect samples of the river water, beginning at the Hindiya Barrage, going through the Hindiya district, and ending at the Al-Kifl district, as shown in figure 1.
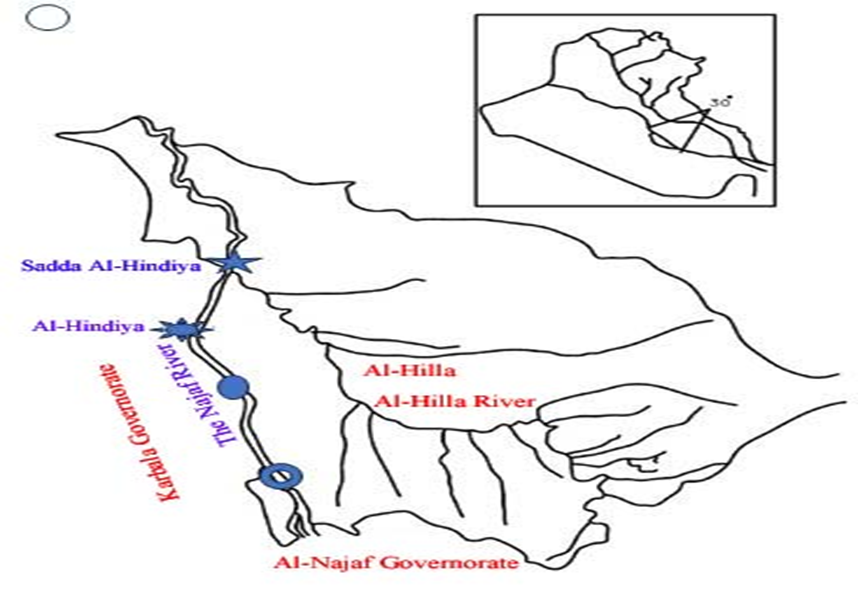
Fig. 1. The Iraqi map by google earth
3. Physical and Chemical Water Tests
3.1 Temperature
Air and water temperatures were measured directly in the field using a standard thermometer graded from 0-100°C.
3.2 PH
The pH value was measured in the field using a pH meter after calibrating with standard buffer solutions (4, 7, and 9) [13].
3.3 Electrical Conductivity
In the field, the electrical conductivity of the water was assessed using a portable conductivity meter (HI 9811-0, HI 9811-5) manufactured by HANNA. The results were quantified in microsiemens per centimeter, and the salinity values were determined using conductivity measurements [13].
3.4 Total Dissolved Solids (TDS)
Total dissolve solid were measured directly in the field using a TDS meter (HI 9811-0, HI 9811-5) by HANNA, with results expressed in mg/L [13].
3.5 Total Hardness
Total hardness was determined by titration with 0.01 EDTA-2N solution using Eriochrome Black T as an indicator, with results expressed in mg/L [13].
3.6 Calcium Hardness
Calcium hardness was determined by titration with EDTA-2Na solution and 1 N NaOH, using Murexide as an indicator, with results expressed in mg/L [14, p. 1-30].
3.7 Magnesium Hardness (mg L-1)
Magnesium values were calculated using the formula:
mg Mg+2 / L [mEq hardness /L - mEqCa+2/L] x 12.16
mEq hardness /L = mg hardness] x 0.0499
mEqCa +2 = mgCa+2 ] x 0.0499
3.8 Chlorides
25-50 ml of river water was placed in a conical flask, a few drops of potassium chromate were added until a yellow color formed, and titration was done with silver nitrate until a red color appeared. The result was calculated from the burette reading [14, p. 1-30].
4. Algae Study
4.1 Qualitative Study
Non-diatom algae were examined under a microscope at 1000x magnification (Olympus) after thorough shaking of the sample bottle. Diatoms were clarified using nitric acid and examined at 40-100x magnification.
4.2 Quantitative Study
The method by Furet and Benson-Evan (1982) was followed. 25 ml of sample water was placed in a 100 ml graduated cylinder, topped up to 100 ml, and 1 ml of Lugol's solution was added for sedimentation over ten days. The top 90 ml was removed using a vacuum device, and the remaining 10 ml was used for quantitative and qualitative algae assessment.
5. Preparation of Diatom Slides and Cell Counting
A clean hemocytometer slide is taken and placed on a hot plate (70°C). Using a fine pipette, 0.05 ml of the well-mixed concentrated sample is placed in the center of the glass slide. The drop is left to dry completely. A drop of concentrated nitric acid is then placed in the center of the dried drop. After the acid evaporates, a small amount of Canada balsam is taken and placed on a cover slip, which is then inverted onto the dry drop with gentle pressure to ensure the even spread of the Canada balsam and to avoid air bubbles near the edges of the cover slip. The slide is then ready for counting [15].
6. Results
6.1 Physical and Chemical Water Tests
6.1.1 Temperature of Air
According to figure 2, the air temperature fluctuated between 15°C and 49°C throughout the study period from November to August 2023. Stations 1 and 2 reported the coldest air temperatures in January, March, and December, respectively. August recorded the greatest air temperatures at stations 4 and 3, reaching 47.1°C and 49.6°C, respectively. The analysis findings revealed statistically significant disparities in air temperature among the sample stations and months, with a significance level of 0.05.
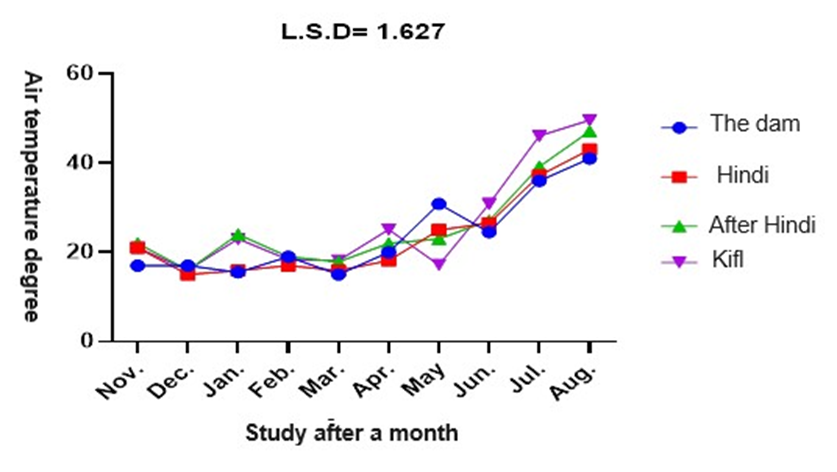
Fig. 2. Monthly variation in temperature of air at study stations. Water temperature
6.1.2 Water Temperature
According to figure 3, the water temperature at study sites 1, 2, 3, and 4 varied from 12°C to 39°C. The minimum water temperature of 12°C was documented in December at station 1, and the maximum water temperature of 39°C was recorded in August at station 3. The water temperature at stations 1 and 2 fluctuated between 18°C and 38°C for the whole duration of the study period. Figure 7 demonstrates notable statistical disparities in water temperature among the stations and study months, with a significance level of 0.05. Presented below is the temperature chart:
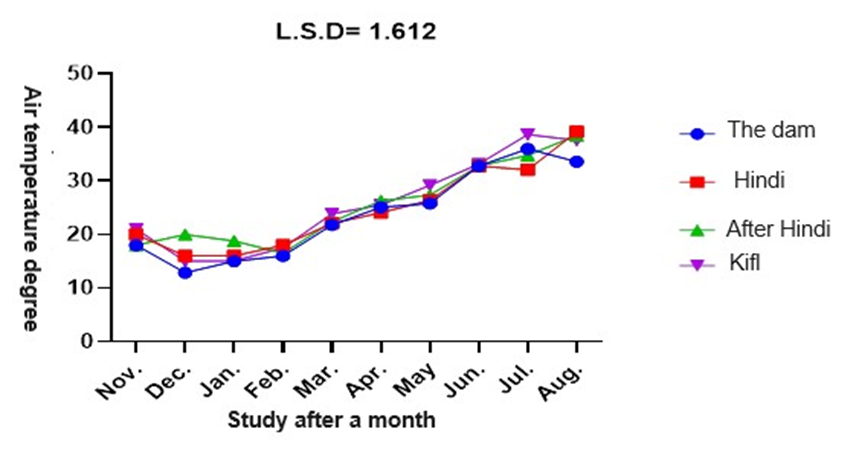
Fig. 3. Monthly variation temperature of water at study stations. pH (Hydrogen ion concentration)
6.1.3 pH Values
The data presented in figure 4 show that the pH values varied across the stations and study months (from November to August 2023). These variations included both spatial changes (among stations) and temporal changes (across months). However, these changes were not statistical significant at a probability level of 0.05. The highest pH value recorded was 8.6 at station 1 in November, while the lowest pH value recorded was 7.1.
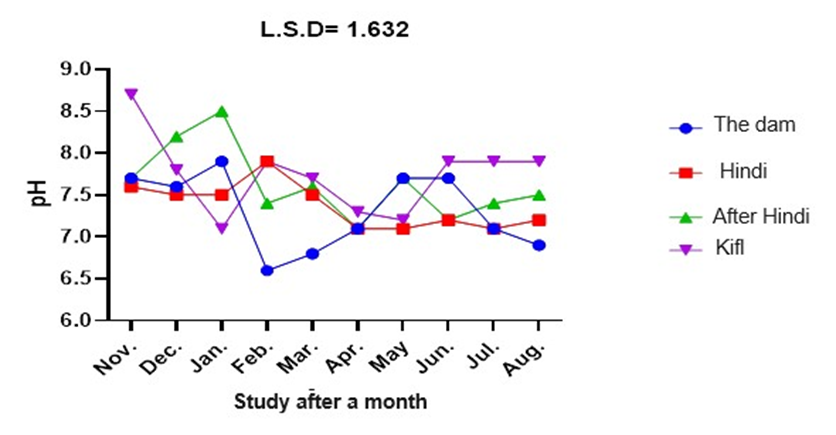
Fig. 4. Monthly variations of pH at the study stations
6.1.4 Electrical Conductivity (EC)
According to figure 5, station 4 had the highest average electrical conductivity values at 1555.70 mg/L, which were significantly different from the values obtained at stations 1, 2, and 3. Nevertheless, there was no substantial statistical disparity observed in the electrical conductivity measurements obtained at stations 1 and 2. The data also shows notable disparities in electrical conductivity levels measured across the months of the study. Station 2 had the highest electrical conductivity (EC) value in June, while station 4 had the lowest EC value in November, and station 3 had the lowest EC value in August.
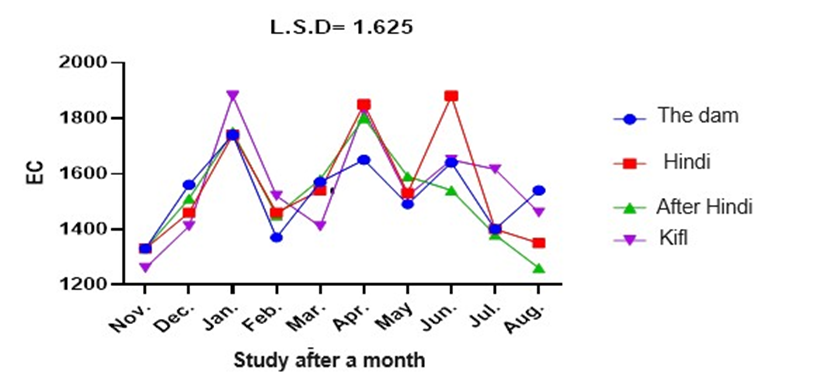
Fig. 5. For monthly electrical conductivity at the study stations
6.1.5 Total Dissolved Solids (T.D.S.)
The data presented in figure 6 indicate that the total dissolved solids (T.D.S.) values varied from 650 mg/L to 860 mg/L at station 1 over the course of the research months. However, it should be noted that these differences were not found to be statistically significant. At station 2, the Total Dissolved Solids (T.D.S.) values varied between 660 mg/L and 930 mg/L, and there were noticeable variations throughout the course of the study months. The T.D.S. values at stations 3 and 4 varied between 650 mg/L and 901 mg/L, and between 690 mg/L and 910 mg/L, respectively. Furthermore, there were no statistically significant variations in T.D.S. values among all stations and study months at a significance level of 0.05.
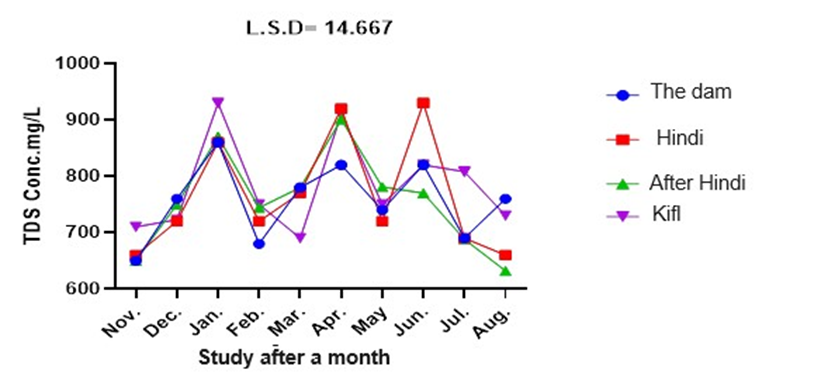
Fig. 6. Monthly variations of total hardness at the study stations
6.1.6 Total Hardness
According to figure 7, the total hardness measurements reveal that the greatest value of total hardness was seen at station 1, measuring 615 mg/L. This value was considerably greater than the values reported at the other stations. The maximum monthly value was 755 mg/L, seen in July, which exhibited a substantial deviation from the values reported in the remaining months of the study. The total hardness values varied from 516 mg/L to 840 mg/L at station 1, from 360 mg/L to 760 mg/L at station 2, from 364 mg/L to 728 mg/L at station 3, and from 440 mg/L to 900 mg/L at station 4 (Al-Kifl) during the study period. There were notable variations in overall hardness values among the several stations and over the course of the research months, with statistical significance at a level of 0.05.
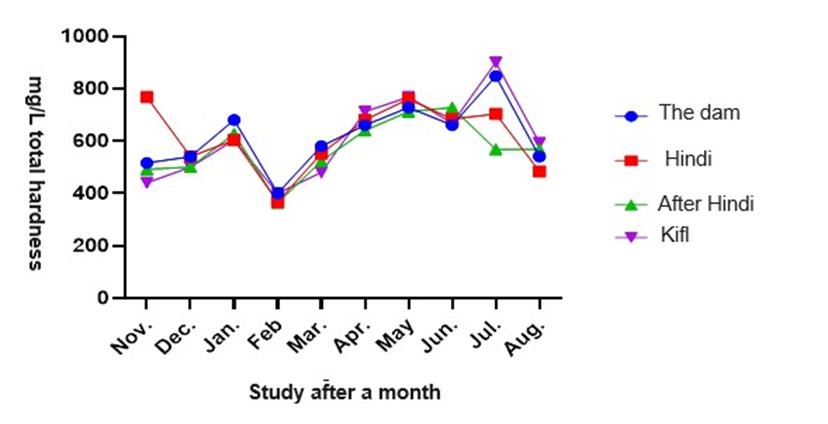
Fig. 7. Shows monthly variations in total hardness values at the study stations
6.1.7 Calcium Hardness
The calcium hardness levels exhibited variability across different research locations and months, as depicted in figure 8. The calcium hardness levels at stations 1 and 3 varied between 364 mg/L and 900 mg/L in February and June, respectively. The calcium hardness levels at stations 1 and 2 varied between 364 mg/L and 760 mg/L from February to November. These differences were statistically significant with a p-value of 0.05.
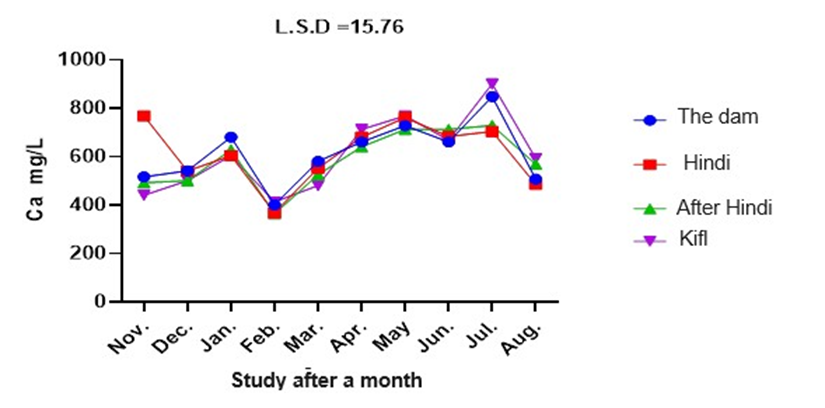
Fig. 8. Shows monthly variations in calcium hardness values at the study stations
6.1.8 Magnesium Hardness
The magnesium hardness values varied from 168 mg/L to 764 mg/L at station 1, from 130 mg/L to 86 mg/L at station 2, from 145 mg/L to 67 mg/L at station 3, and from 178 mg/L to 70 mg/L at station 4. There were statistically significant variations in the levels of magnesium hardness between the months of November and August in the study, with a significance level of 0.05. Moreover, there were notable disparities among the stations in relation to the levels of magnesium hardness. Station 2 reported the highest value of 119.0 mg/L, whilst station 3 recorded the lowest value of 110.0 mg/L. Moreover, the duration of the study had a notable influence on the levels of magnesium hardness, with the peak value observed in July and the minimum value in February, as depicted in figure 9.
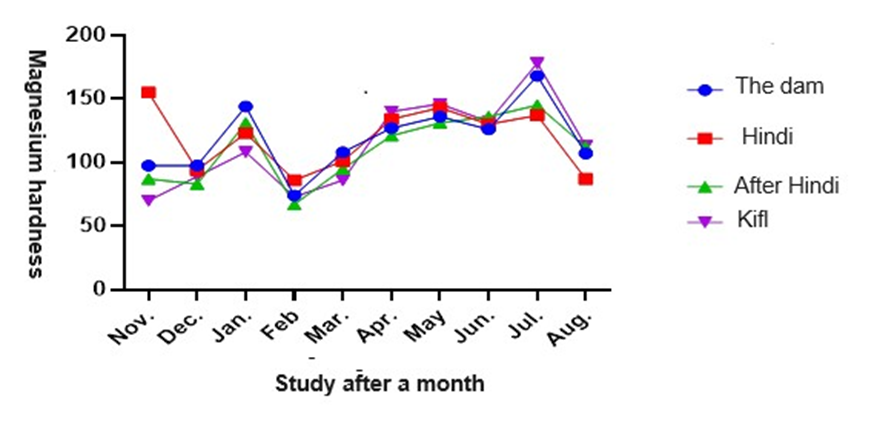
Fig. 9. Shows monthly variations in Magnesium hardness values at the study stations
6.1.9 Chlorides
Figure 10 shows that there is a significant impact of the study stations and months on chloride concentrations from January to August. Station 4 recorded the highest chloride concentration of 342.0 mg/L in January, while station 1 recorded the lowest chloride concentration of 170.0 mg/L in August. The chloride concentrations at stations 2 and 3 ranged between 180.0 to 284.0 mg/L and 182.0 to 243.0 mg/L, respectively. There were significant temporal and spatial differences in chloride concentrations at a significance level of 0.05.
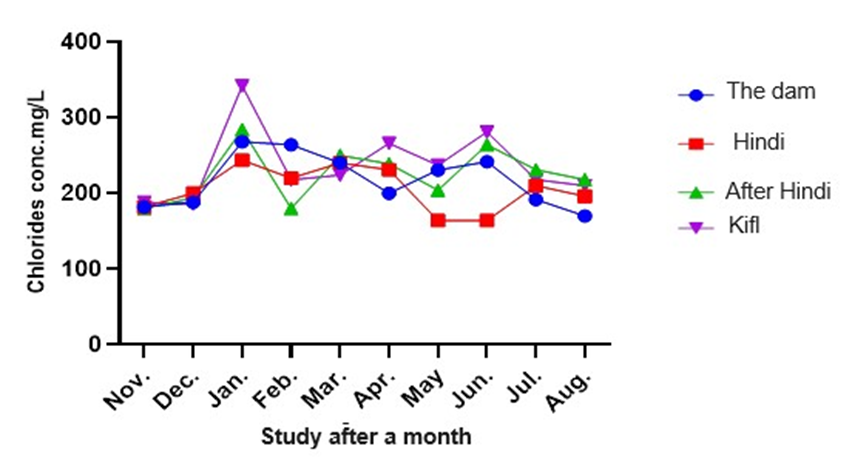
Fig. 10. Shows monthly variations in chloride values at the study stations
Algae demonstrate remarkable resilience and adaptability to various environmental stresses, enabling them to survive under diverse conditions, including fluctuating temperatures during the study months. The results indicate a significant impact of the study locations on algae counts, with the highest average number of algae being 195.5 cells/cm³ at station 3, while the lowest count was 122 cells/cm³ at station 4.
The data also show a significant influence of the study months on algae counts. The highest average count of 252 cells/cm³ was recorded in June, compared to the lowest average of 95 cells/cm³ in December. Algae counts varied significantly across the other months, ranging between 101.5 and 237.88 cells/cm³.
Additionally, the type of plant significantly affected algae counts. The algae count for Ceratophyllum demersum was 201.85 cells/cm³, while for Phragmites australis; the count was lower at 129.54 cells/cm³, with a statistically significant difference.
7. Epiphytic Algae on Phragmites australis
The results indicate that the highest value for algae on Phragmites australis was recorded in November at station 1, with 393.7 cells/cm³. The minimum value was recorded in December at station 3, with 13.0 cells/cm³.
8. Epiphytic Algae on Ceratophyllum demersum
The statistical results showed in table 1 the highest value in June at station 3, with 557.0 cells/cm³. The minimum value were recorded in November at station 2, with 101.5 cells/cm³.
Table 1
The effect of stations, months, and plants on the numbers of algae per unit cell/cm3
| Months | Plant | stations | |||
| 1 | 2 | 3 | 4 | ||
| November | Phragmites australis | 393.7 | 48.0 | 179.0 | 59.0 |
| Ceratophyllum | 96.0 | 23.0 | 287.0 | 150.0 | |
| December | Phragmites australis | 75.0 | 24.0 | 13.0 | 113.0 |
| Ceratophyllum | 211.0 | 211.0 | 77.0 | 36.0 | |
| January | Phragmites australis | 110.0 | 310.0 | 177.0 | 44.0 |
| Ceratophyllum | 155.0 | 180.0 | 288.0 | 81.0 | |
| February | Phragmites australis | 166.0 | 101.0 | 322.0 | 153.0 |
| Ceratophyllum | 410.0 | 213.0 | 380.0 | 158.0 | |
| March | Phragmites australis | 177.0 | 191.0 | 140.0 | 99.0 |
| Ceratophyllum | 235.0 | 290.0 | 370.0 | 212.0 | |
| April | Phragmites australis | 165.0 | 75.0 | 67.0 | 38.0 |
| Ceratophyllum | 153.0 | 302.0 | 255.0 | 401.0 | |
| May | Phragmites australis | 68.0 | 155.0 | 103.0 | 202.0 |
| Ceratophyllum | 309.0 | 299.0 | 151.0 | 97.0 | |
| June | Phragmites australis | 87.0 | 92.0 | 212.0 | 153.0 |
| Ceratophyllum | 320.0 | 398.0 | 557.0 | 197.0 | |
| July | Phragmites australis | 88.0 | 258.0 | 71.0 | 33.0 |
| Ceratophyllum | 195.0 | 81.0 | 41.0 | 45.0 | |
| August | Phragmites australis | 109.0 | 143.0 | 91.0 | 77.0 |
| Ceratophyllum | 98.0 | 199.0 | 122.0 | 111.0 | |
| L.S.D 0.05 | 1.63 | ||||
| Effect of plant species | Phragmites australis | Ceratophyllum | |||
| 129.54 | 209.85 | ||||
| L.S.D 0.05 | 0.26 | ||||
9. Algae
A total of 174 species were identified in this study in table 2, belonging to five classes as shown in Figure 11. The distribution of these species is as follows:
Bacillariophyceae (Diatoms): This class constituted 60.34% of the total identified species. Among these, 51.14% were recorded on Phragmites australis, with 89 species identified on Ceratophyllum demersum (48.27%) and 84 species on Phragmites australis.
Chlorophyceae (Green Algae): This class represented 21.83% of the total species. On Ceratophyllum demersum, they accounted for 17.81%, and on Phragmites australis, they made up 17.24%.
Cyanophyceae (Blue-Green Algae): These algae formed 16.66% of the total species. On Ceratophyllum demersum, they constituted 14.94%, and on Phragmites australis, they made up 10.91%.
Euglenophyceae: Only one species was identified in this class, representing 0.57% of the total. This species was recorded on both Ceratophyllum demersum and Phragmites australis, each making up 0.57%.
Dinophyceae: This class also constituted 0.57% of the total species. One species was identified on Ceratophyllum demersum, but none were found on Phragmites australis.
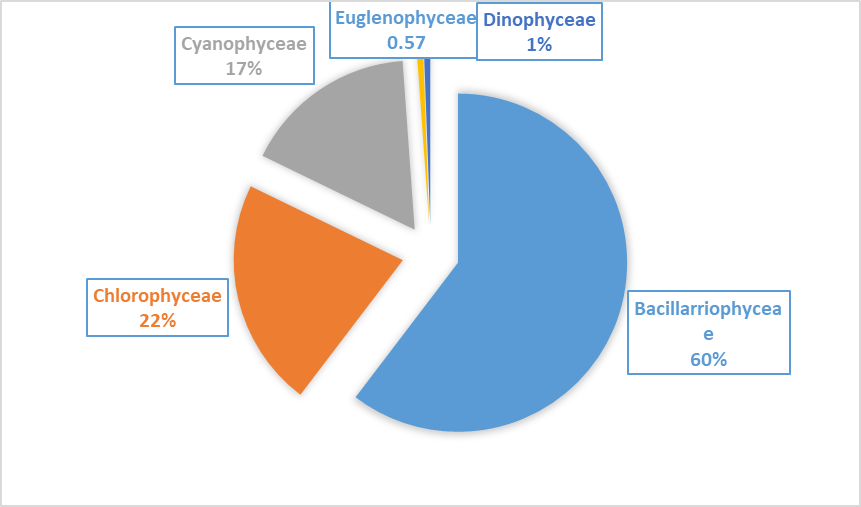
Fig. 11. Shows monthly variations in classes of Algae
Table 2
A list of the types of algae identified in the four studied sites for both the reed and shamrock plants during the months of the study (+ type present and - type not found)
Cyanophycea | Epiphytic | |
|---|---|---|
P.australis | C.demersum | |
| Anabaena Sp. | + | + |
| Calothrix Sp. | - | + |
| C. turgid us (Ktz.) Naegeli | + | - |
| C. minutes (Ktz.) Naegeli | + | + |
| Gloecapsa sp | + | + |
| Lyngbya arboricola Bruhl et Biswas | - | + |
| L.mesotrica Skuja | + | + |
| Lyngbya sp. | - | + |
| M. glauca (Ehr.) Naegeli | + | - |
| M.minima Heck | + | - |
| L.major Meneghini | - | + |
| Nostok Sp. | + | + |
| Oscillatoria animelis Agardh | + | - |
| O. articulata Gardner | + | + |
| O. curviceps Agardh | - | + |
| O.limosa Roth Agardh | - | + |
| O. nigra Vaucher | - | + |
| O. rubescens de Candoll | + | - |
| O.sancta (Ktz.) Gomont | - | + |
| O. tenuis Agardh | + | - |
| Oscillatoria SP. | + | + |
| Phormidium tenue (Menegh) Gom.Gomont | - | + |
| P.luciduis Ktz | + | - |
| Phormidium Sp. | + | + |
| Spirulina Laxa G.M.Smith | + | - |
| S.major Ktz. | + | + |
| S. princeps West and West | + | + |
| Chlorophyceae | ||
| Ankistrodesmus convolus Corda | + | + |
| Asterococcus superbus (Cienk) Scherffel | - | + |
| Chlamydomonas angulosa Dill | + | - |
| C.epiphytica G. M. Smith | + | + |
| C. globosa Snow | + | - |
| Chlamydomonas sp. | + | + |
| Chlorella vulgaris Bejerinck | - | + |
| Chlorococcum humicola Naeg. | + | - |
| Cladophora Sp. | + | + |
| Closterium parvalum Naegeli | - | + |
| Coelostrum astoideum De Not | + | - |
| Cosmarium botrytis Meneghinii | + | + |
| C. leave Rabenhorst | + | + |
| Gonium pectoral Mueller | - | + |
| Mougeotia boodle | - | + |
| Oedogonium sp. | + | - |
| Pediastrum boryanum (Turp.) Meneghini | + | - |
| P. duplex Meyen | + | + |
| P. simplex Meyen | + | + |
| Scenedesmus aboundans (Kirch) Chodat | + | + |
| Scenedesmus acuminatus (Lag.) Chodat | + | + |
| S. armatus Chodat | + | + |
| S. bernardii Smith | - | + |
| S.bijuga (Turb.) Lagher | + | + |
| S.dimorphus (Turb.) Ktz. | - | + |
| S. quadricauda (Turb.) de Brebisson | + | + |
| S. quadricauda var westii | + | + |
| Selanastrum gracile (Reinsch) Korsch | - | + |
| Selanastrum sp. | + | + |
| Spirogyra longata (Vauch.) Kuetzing | + | + |
| Spirogyra sp. | + | + |
| Staurastrum alternans | + | + |
| Staurastrum sp. | + | + |
| Tetraedron hastatum (Reisch) Hansg. | + | + |
| T.regulare Ktz. | + | + |
| Ulothrix zonata (Webre and Mohr.) Ktz. | + | - |
| Ulothrix sp | + | + |
| Zygnema sp | + | + |
| Euglenophyceae | ||
| Euglena gracilis Klebs | + | + |
| Dinophyceae | ||
| Ceratium hirundinella (Muell.) Du Jardin | - | + |
| Bacillariophyceae | ||
| Order Centrale | ||
| Coscinodiscus lacutirs | + | + |
| C. comta (Ehr.) Kuetzing | + | + |
| C. meneghiniana Kuetzing | + | + |
| C.ocellata Pentose | + | + |
| M. granulate (Ehr.) Ralfs | + | + |
| M.varians Agradh | + | + |
| Stephanodiscus astrea (Ehr.) Grun | + | + |
| S. dubius (Fricke) Hustedt | + | + |
| Order Pennales | ||
| Amphora coffeaeformis (Ag.) Kuetzing | + | + |
| A. normannii Rab. | - | + |
| A.ovalis (Ktz.) Kuetzing | + | + |
| Amphora veneta Kuetzing | + | + |
| Bacillaria faxillifer (Muell.) Hendey | + | + |
| Caloneis amphisbaena (Bory) Cleve | + | + |
| C.permagna (Bail.) Cleve | + | - |
| Caloneis ventricose (Ehr.) Meister | + | - |
| Cocconeis pediculus Ehernberg | + | + |
| C.placentula Ehernberg | + | + |
| Cymatopleura elliptica (Berb.) W.Smith | + | + |
| C. solea (Berb.) W. Smith | + | + |
| Cymbella affinis Kuetzing | + | + |
| C. amphicephala Naegeli | + | + |
| C. aspera (Ehr.) H.paragallo | + | + |
| C. caepitosa Kuetzing | + | + |
| C. cistula (Ehr.) Kirchn | + | + |
| C. delicatula Kutz. | + | + |
| C. gracilis | + | + |
| C. helvetica Kuetzing | + | + |
| C. lanceolata (Ehr.) | + | + |
| C. leptoceros (Ehr.) Grunow | + | + |
| C. parva (W. Smith) Kitchn | + | + |
| C. tumida (Berb.) van Heurck | + | + |
| C. tumidula Grunow | + | + |
| C. turgid (Greg.) Cleve | + | + |
| Diatoma elongatum (Lyngb.) gradhA | + | + |
| D. hiemale (Roth.) Heiberg | + | + |
| D. vulgare Bory | + | + |
| Diploneis ovalis (Hilse) Cleve | + | + |
| D. smithii (Berb.) Cleve | + | + |
| Eutonia curvata | - | + |
| E. pectinalis (Ralfs) Rabenhorst | + | + |
| Fragilaria bervistriata Grunow | + | + |
| F. capucina Desmazieres | + | + |
| F. virescens Ralfs | + | + |
| Gomphoneis olivaceum (Horne) P. Dawson ex Ross et Sims | + | + |
| G. acuminatum Ehernberg | + | + |
| G. angustatum (ktz.) Rabenhorst | + | + |
| G. constrictum Ehernberg | + | + |
| G. fanensis Maillard | + | + |
| G. gracile Ehernberg | + | + |
| G. intricatum Kuetzing | + | - |
| G. parvulum (ktz.) Kuetzing | + | + |
| G. tergestinum (Grun.) | + | + |
| Gyrosigma acuminatum (ktz.) Rabenhorst | + | + |
| G. attenuatum (ktz.) Rabenhorst | - | + |
| Hantzschia amphioxys (Ehr.) Grunow | + | - |
| Mastogloia elliptica (Ag.) Cleve | + | + |
| M. smithii Thw. Ex. W. Sm | - | + |
| Navicula. anglica Ralfs | - | + |
| N. cincta (Ehr.) | + | + |
| N. gibbula Cleve | + | + |
| N. gracilis (Ehr.) | + | + |
| N. graciloides A.Mayer | + | - |
| N. halophila (Grun.) Cleve | + | + |
| Neidium affine (Ehr.)Pfitz | + | + |
| Nitzschia acicularis (ktz.) W. Smith | + | + |
| Ni. Hantzsch | + | + |
| Ni. amphibia Grunow | + | + |
| Ni. commutata Grunow | + | + |
| Ni. Dissipata (ktz.) Grunow | - | + |
| Ni. fruticosa Grunow | - | + |
| Ni. gracilis Hantzsch | + | + |
| Ni. hantzschiana Rabenhorst | + | + |
| Ni. hungarica Grunow | + | + |
| Ni. intermedia Hantzsch ex Cleve et Gran. | + | + |
| Ni. longissima (Berb.) Ralfs | + | + |
| Ni. obtusa W. Smith | + | + |
| Ni. palea (ktz.) W. Smith | + | + |
| Ni. parvulla W. Smith | + | + |
| Ni. recta Hantzsch ex Rabenh. | + | + |
| Ni. romana Grunow | + | + |
| Ni. sigma (ktz.) W.Smith | + | + |
| Ni. sigmoidea (Ehr.) W. Smith | + | + |
| Ni. tryblionella Hantzsch | + | + |
| Nitzschia Sp. | + | + |
| Pinnularia acrosphaeria de Brebisson | + | - |
| P. divergins Ehr. | - | + |
| P. gibba Ehr. | - | + |
| P. viridis (Nitzsch.) Ehrenberg | + | - |
| Pinnularia Sp. | + | + |
| Rhoicosphenia curvata (ktz.) Grunow | + | + |
| Rhopalodia gibba (Ehr.) O. Mueller | - | + |
| R. gibberula (Ehr.) O. Mueller | + | - |
| R. musculus Kuetz | - | + |
| Rhopalodia sp. | + | + |
| Surirella ovalis de Brebisson | + | + |
| S. ovate Ktz. | + | + |
| S. tenera Gregory | + | + |
| Synedra acus Kueting | + | + |
| S. capitata Ehrenberg | - | + |
| S. pulchella (Ralfs) Kuetzing | + | + |
| S. ulna (Nitzs.) Ehrenberg | + | + |
| S. ulna var. oxyrynchus (Ktz.) Van Heurck | + | + |
| Tryblionella coarctata | + | + |
| T. levidensis | - | + |
10. Discussion
The present investigation documented substantial disparities in the quantity of genera and species recognized across all locations. The tested areas of the Euphrates River exhibited a significant abundance and variety of epiphytic algae on Phragmites australis and Ceratophyllum demersum. The density of these algae is influenced by seasonal fluctuations and the nutrient composition of the Euphrates River, which includes nitrates, phosphates, and carbon dioxide (CO2) required for photosynthesis [16, p. 605-614; 17]. These algae function as indicators of pollution in river water and as the main producers in aquatic food chains. Multiple studies have demonstrated that epiphytic algae serve as a reliable indicator of external factors affecting water quality, surpassing the effectiveness of phytoplankton [18, p. 57-63]. Station 3 exhibited the greatest abundance of phytoplankton, whilst station 4 had the lowest count. The findings also indicated notable monthly fluctuations, with the highest mean algal count observed in June and the lowest in December, demonstrating statistically significant variances over the study period. Ceratophyllum demersum exhibited a greater abundance of algae compared to Phragmites australis, with a statistically significant distinction at a probability level of 0.005. The elevated levels of algae seen on Ceratophyllum demersum for the whole duration of the study can be attributed to its consistent presence throughout the year, which allows for sufficient time for algal proliferation. Multiple studies conducted in different aquatic settings in Iraq, such as the Tigris and Euphrates rivers, have demonstrated that diatoms surpass other types of algae in abundance due to their exceptional ability to withstand challenging environmental circumstances [19, p. 39-50]. The studies conducted in the Tigris and Euphrates rivers on epiphytic algae revealed that diatoms were the most prevalent, followed by green algae [19, p. 39-50; 20, p. 495-505].
Diatoms have a higher numerical density than other algae that grow on water plants, as documented in numerous local (Iraqi) and global studies [9, p. 37-52]. Environmental elements, such as light and temperature, play a vital role in the growth and attachment of algae to aquatic plants like Phragmites australis and Ceratophyllum demersum. This attachment occurs in several areas of the plants, including the stems, roots, and leaves. The inclusion of nutrients such as nitrates, phosphates, nitrogen, and silica also has a notable impact [21, p. 421-438; 22]. Typically, during the summer season, the density of algae tends to rise as a result of increased levels of light, temperature, and nutrients. These factors create optimal circumstances for the growth of algae [23, p. 18-32]. During the winter season, there is a decrease in the abundance of algae, especially green and blue-green algae. This is because of the colder temperatures, reduced sunlight, and increased river water levels caused by rainfall [24, p. 1443-1451]. The present investigation revealed a reduction in the concentration of green and blue-green algae throughout the winter season at all locations as a result of dredging operations and the combustion of plants in the study area. This decline led to a drop in the overall number of epiphytic algae on Phragmites australis and Ceratophyllum demersum. Furthermore, the loss was exacerbated by the elevated water levels in the Euphrates River resulting from substantial precipitation and snowfall [25, p. 1-14]. The findings revealed a significant resemblance in the quantity of diatom species adhering to the same host plant across several seasons. This implies that the host plant's geometric shape, kind, and environmental circumstances have an impact on the makeup of the epiphytic algal population. The greatest resemblance between diatoms found on plants was observed in the Chlorophyceae group on Ceratophyllum demersum and Phragmites australis. The greatest resemblance in host plants was observed in Euglenophyceae diatoms found in both Ceratophyllum demersum and Phragmites australis. In contrast, Bacillariophyceae diatoms exhibited the lowest similarity between these plants, with a larger abundance on Ceratophyllum demersum. The variation could be attributed to the structural characteristics of the host plant and the prevailing environmental factors in the Euphrates River. The prevalence of algae species from the genera Cocconeis, Achnanthes, Nitzschia, Gomphonema, Navicula, Cymbella, Synedra, Cyclotella, and Oscillatoria on all epiphytic plants during the entire study period probably played a significant role in the observed high similarity, as these are plants that grow underwater. This discovery is consistent with previous research conducted by scholars [26, p. 1-10; 27; 28, p. 1-16], which revealed that the prevalence of a certain type of algae resulted in notable variations in the makeup of epiphytic algae on plants. The Scenedesmus algae's capacity to adhere to Phragmites australis and Ceratophyllum demersum is due to its extensive distribution in Iraqi and global water bodies, consistent nutrient supply, and its ability to withstand adverse conditions. The present study's results align with those of [29, p. 513-519], which demonstrated that the presence and characteristics of epiphytic algae on aquatic plants are impacted by several chemical and physical features of the river, such as the availability of CO2, total alkalinity, pH, and light. These factors have an impact on the quality and overall abundance of the algae. Prior research has ascribed the disparities to fluctuations in environmental factors and seasonal fluctuations. In the present study, diatoms were shown to be the most prevalent algal groupings. This is attributed to their capacity to thrive in many aquatic habitats and their structural adaptations for attachment, such as stalks in Gomphonema and gelatinous sheaths in Cymbella and Navicula, or by forming colonies. Diatoms possess characteristics that provide them a competitive advantage and promote their growth on aquatic hosts, distinguishing them from other types of algae [30, p. 5878-5883; 31]. The prevalence of high silica concentrations in the algae's environment also contributes to their dominance [32]. Diatoms are the most common type of algae that grow on the surface of plants in the seas of Iraq [33; 34]. Their dominance in running is evident [35, p. 1510-1533]. The present investigation revealed that Dinophyceae is the least abundant class of algae, with just one species observed on Ceratophyllum demersum and none on Phragmites australis. This difference is attributed to factors such as the duration of plant growth, the horizontal position in the water, and the smaller percentage of Phragmites australis that is submerged compared to totally submerged Ceratophyllum demersum [36, p. 10-18].
11. Physical and Chemical Parameters
The temperature is vital because it affects the presence of aquatic life and the biological functions of aquatic species. It has a substantial impact on the process of dissolving elements and gasses in the aquatic environment [37; 38, p. 1443-1451]. Temperature fluctuations impact the physiology, behavior, and dispersion of living organisms. The present study documented distinct monthly fluctuations in water and air temperatures, characterized by elevated temperatures during the summer months and reduced temperatures in winter, which can be related to Iraq's climate. The temperature differences seen among the study locations can be attributed to changes in the sampling time and depth [39, p. 90-100]. Temperatures start off lower in the morning and then rise as we approach midday. In addition, the pace at which water flows can play a role in achieving thorough mixing, resulting in consistent temperatures throughout the water column without any variation in thermal stratification [20, p. 495-505]. The findings of the present investigation are consistent with other prior studies conducted in Iraq [10; 15; 28, p. 1-16]. pH is a quantitative measure of the concentration of hydrogen and hydroxide ions in water, which is essential for maintaining the chemical and biological equilibrium of water. The pH of aquatic environments is affected by respiration and photosynthesis, which in turn affects the physiology and metabolism of aquatic organisms and the availability of components in the water [40, p. 117-122]. Electrical conductivity (EC) quantifies the presence of both positively and negatively charged ions in water [13]. It denotes the electrical conductivity of water, functioning as an indication of dissolved salts. Electrical conductivity (EC) is strongly correlated with the concentration of total dissolved solids (TDS) and tends to rise in regions affected by agricultural and industrial practices. Electrical conductivity (EC) is influenced by water temperature, such that a one-degree Celsius increase results in an increase in EC. Elevated levels of dissolved salts can also lead to an increase in electrical conductivity (EC), which is influenced by the specific kind and concentration of ions present. In moving streams such as rivers, the electrical conductivity (EC) is typically low. This is mainly due to the dissolving of soil salts and organic materials from living creatures [25, p. 1-14]. Rainfall can lead to elevated EC values by causing soil to be carried into rivers, which in turn increases the concentration of dissolved soil salts. EC can be elevated by the strong agitation caused by waves and the upward transport of substances from deeper levels to the surface.
Total Dissolved Solids (TDS) refers to the presence of inorganic salts and trace amounts of organic matter in water. The primary constituents of suspended solids consist of calcium, magnesium, sodium, potassium cations, bicarbonate, and chlorides. The present investigation observed no statistically significant variations in TDS among the sample locations. Station 4 had the greatest recorded value during winter, followed by an observable rise in suspended solid values during spring and summer. However, there were no significant variations across the stations and study months at a 0.05 probability level. The rise in Total Dissolved Solids (TDS) during the summer months can be attributed to the elevated levels of dissolved salts resulting from evaporation. On the other hand, the variations in suspended solids during the spring season are caused by processes such as dilution, sedimentation, and decomposition [42, p. 7649-7665]. The greatest Total Dissolved Solids (TDS) values observed during winter at station 4 can be attributed to the discharge of waste into the river. This conclusion aligns with the results reported by [23, p. 18-32], who documented the highest TDS levels during summer. The elevated Total Dissolved Solids (TDS) levels observed during winter in the Al-Kifl region can be attributed to agricultural practices. This is mostly due to the higher presence of calcium and sodium ions, which significantly contribute to the salinity of the river. The Total Dissolved Solids (TDS) values are higher than the acceptable limits for aquatic life, both at a local and global level, surpassing 500 mg/L [43, p. 429-441]. Hardness is a crucial chemical characteristic that is used to assess the appropriateness of water for both home and industrial purposes. The presence of dissolved carbonates, calcium, magnesium, and sulfates is responsible for the hardness of water. The overall concentration of positive ions is represented by it, and it is regulated by several multivalent ions, primarily calcium and magnesium [40, p. 117-122]. Calcium, which is a main factor in water hardness, decreases the buffer capacity by reducing the solubility of carbon dioxide [44, p. 29-35]. The present investigation revealed notable fluctuations in calcium hardness, with the most elevated measurements observed at station 4 during the month of July and at station 2 in November. Conversely, the lowest values were recorded at stations 1 and 2 in February and November, respectively. These disparities were statistically significant at a probability threshold of 0.005. The concentration of calcium in natural bodies of water is influenced by the kind of soil and the course of the river, with a notable example being the Euphrates River, which passes through calcareous soil abundant in calcium carbonate. Calcium can also be derived via the disintegration of creatures that possess structures abundant in calcium. Organisms may consume calcium ions for the purpose of building structures and facilitating fish reproduction, which can lead to a decrease in calcium ion levels. Additionally, the development of insoluble compounds can also contribute to lower calcium ion levels [14, p. 1-30]. The higher quantities of calcium relative to magnesium seen during the study period can be attributed to many reasons, such as agricultural runoff, industrial discharge, and sewage [45, p. 218]. Magnesium compounds have greater solubility compared to calcium, and the concentration of magnesium is influenced by both water temperature and the presence of dissolved oxygen [46, p. 81954-81969]. Magnesium is a vital element for the production of chlorophyll in algae and aquatic plants. If there is a lack of magnesium, it might result in a decrease in chlorophyll levels [47]. The study's findings are consistent with the results of previous studies [28, p. 1-16; 48].
12. Conclusions
- Diatoms dominated both in quantity and species over other types of algae.
- There were clear variations in some biological, chemical, and physical, characteristic of the Euphrates River water across different study sites and months.
- A strong correlation was found between the number of epiphytic algae on Phragmites australis and Ceratophyllum demersum and the study sites and months, with higher algal counts in summer and lower counts in winter.
Recommendations
- Conduct diagnostic studies on epiphytic algae on aquatic plants at the molecular level, isolating and identifying species abundantly found in the Euphrates River. Physiological studies should be conducted to explain the mechanisms enabling their presence despite extreme environmental conditions throughout the study months.
- Study the spread and diversity of epiphytic algae.

.png&w=640&q=75)